Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with the calculations Data Sheet Table 1: Calorimeter Experimental Setup Data Collected 57.998 > 58.00 1oooc Measurement Mass of metal rod, g Temperature
need help with the calculations
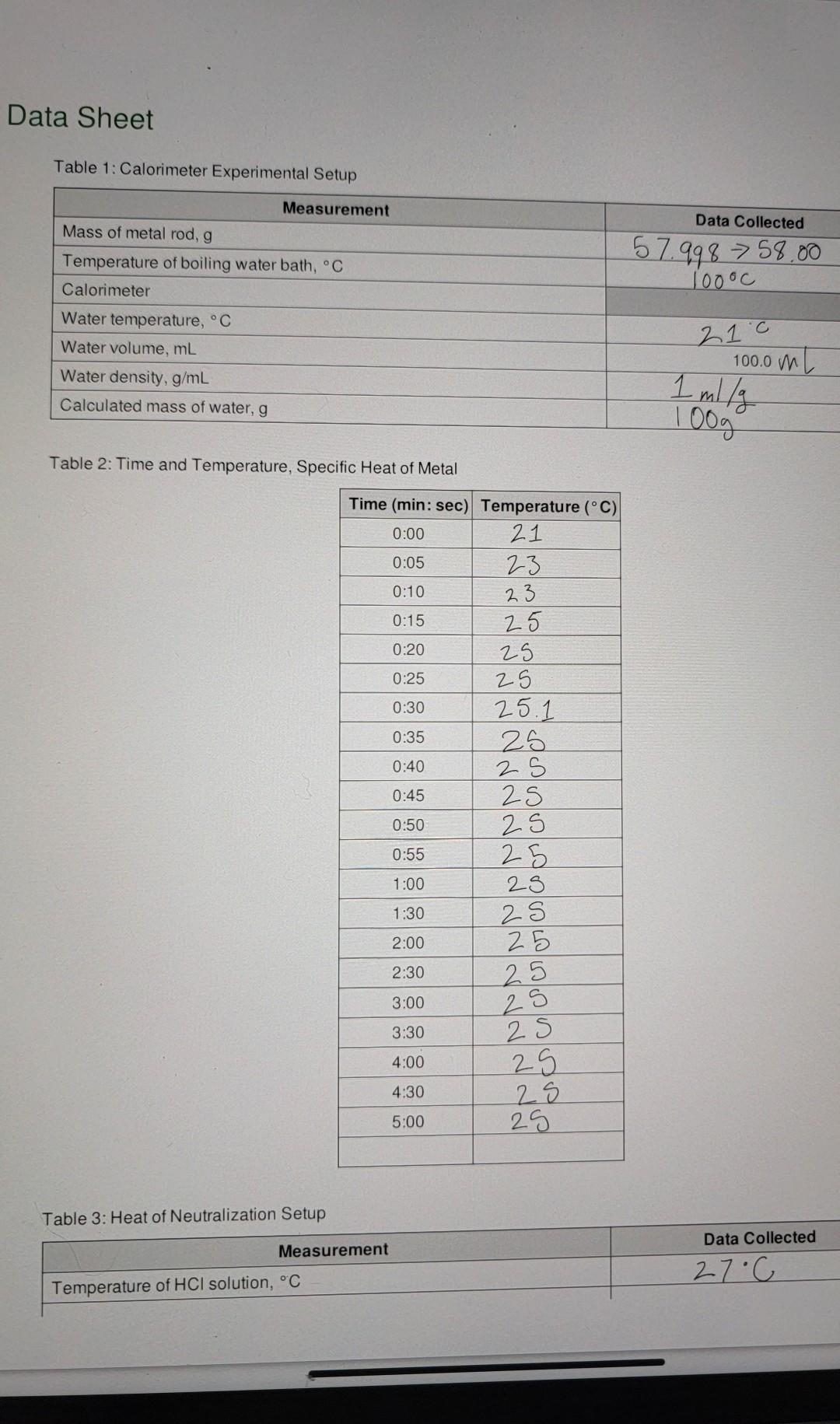
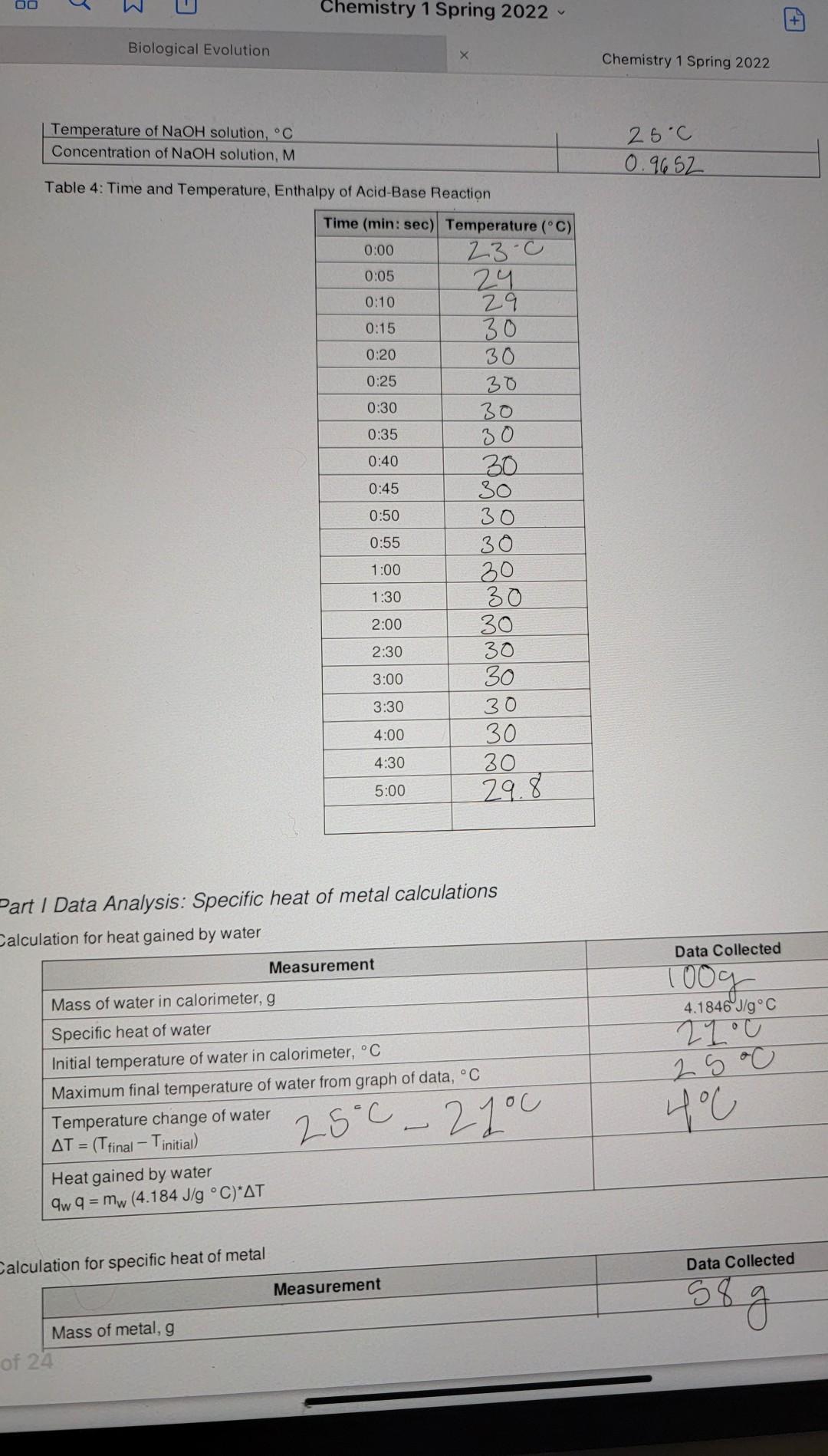
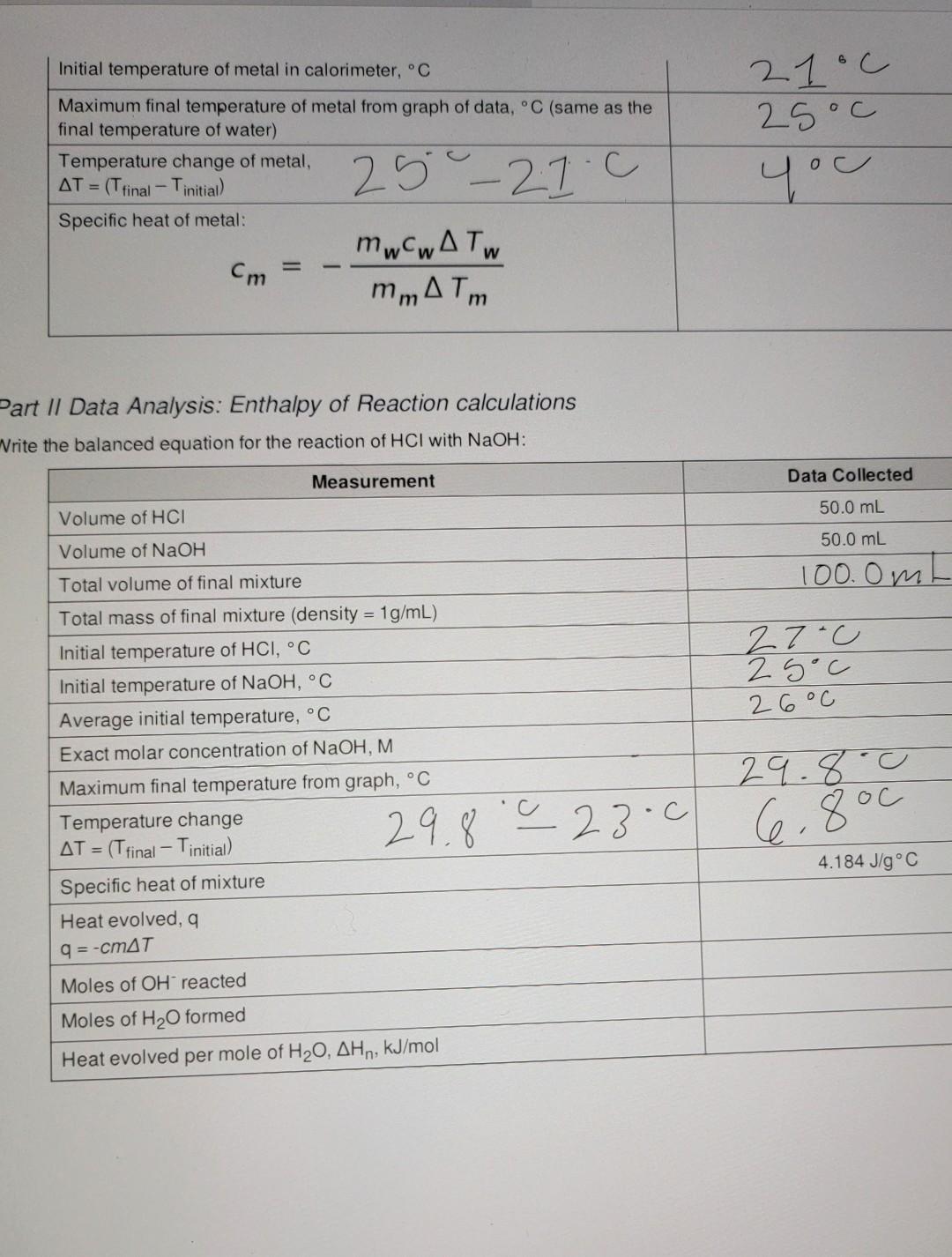
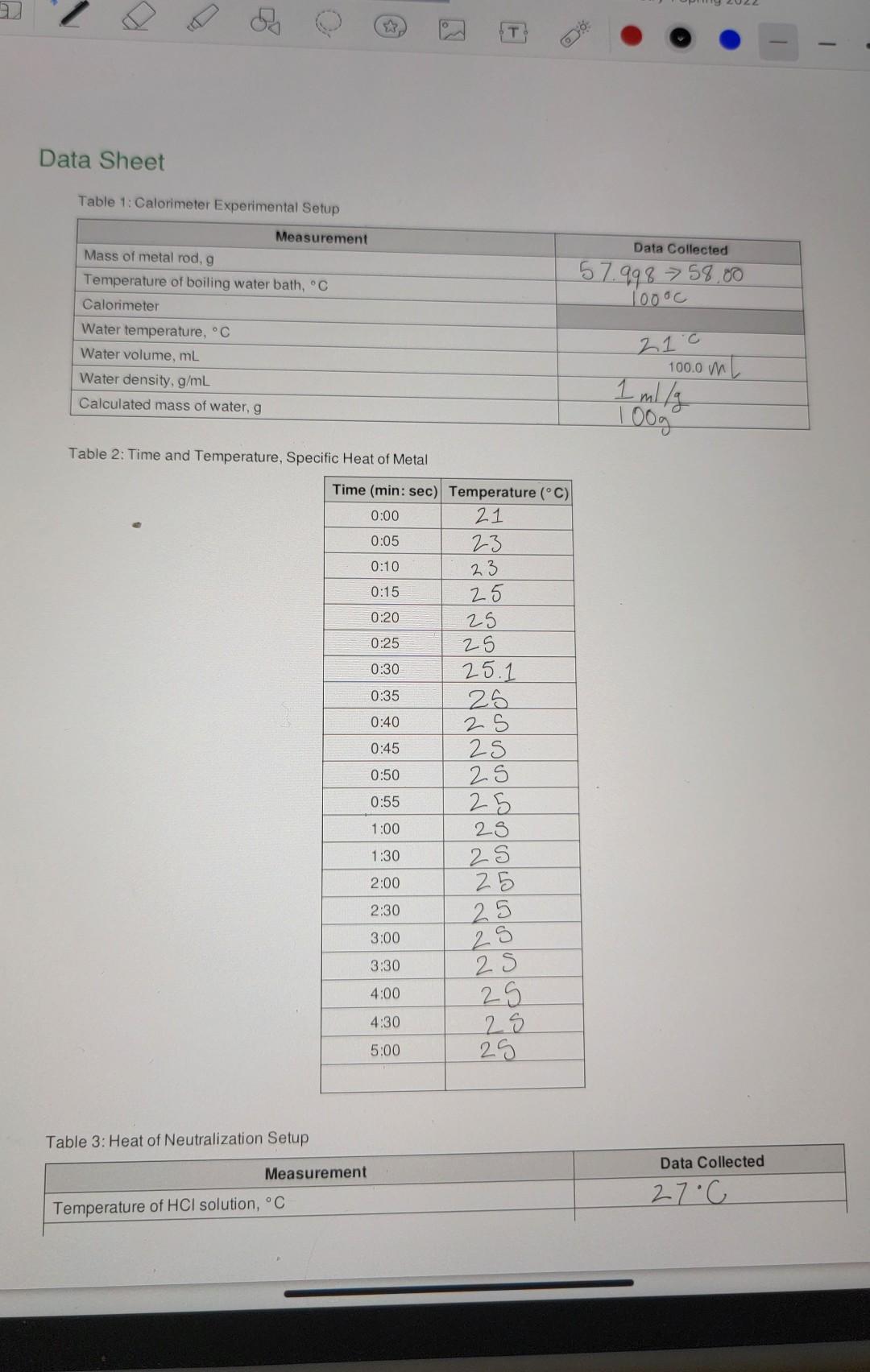
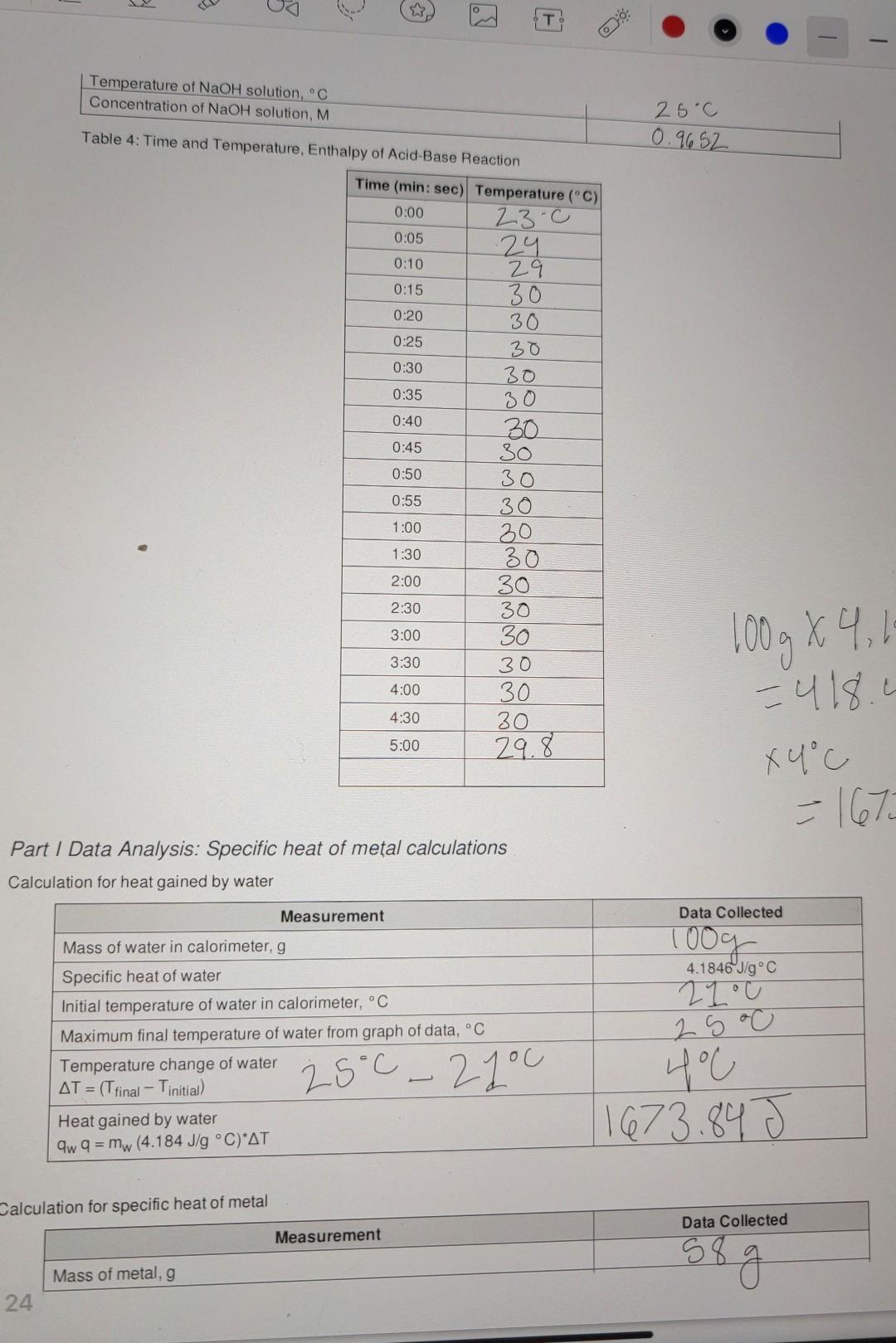
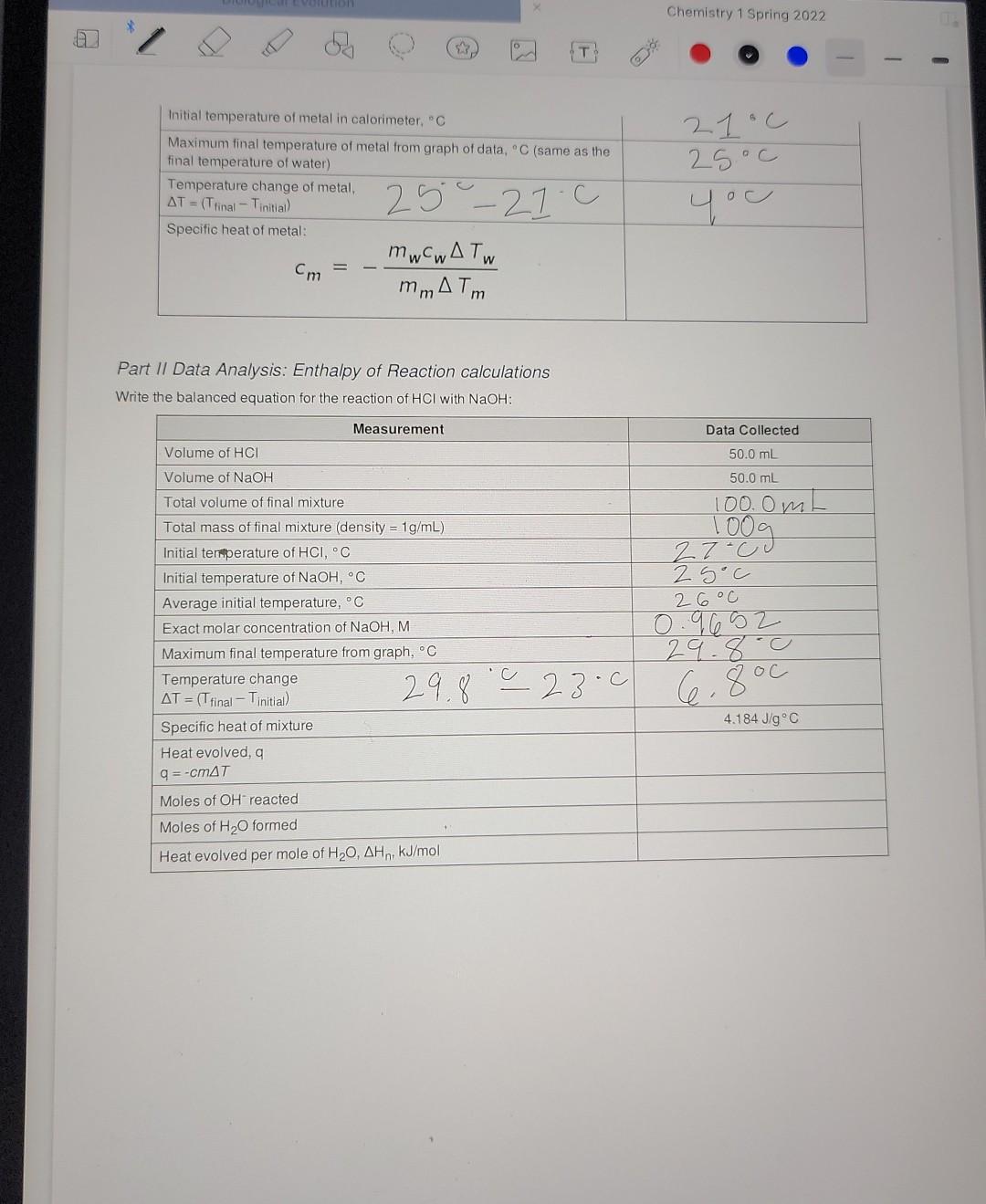
Data Sheet Table 1: Calorimeter Experimental Setup Data Collected 57.998 > 58.00 1oooc Measurement Mass of metal rod, g Temperature of boiling water bath, C Calorimeter Water temperature, C Water volume, mL Water density, g/mL Calculated mass of water, g 21.0 100.0 mL 1 mily 100g Table 2: Time and Temperature, Specific Heat of Metal 0:05 Time (min: sec) Temperature (C) 0:00 21 23 0:10 23 0:15 25 0:20 25 0:25 25 0:30 25.11 0:35 25 0:40 2 S 0:45 25 0:50 2S 0:55 25 1:00 28 1:30 2:00 25 2:30 2S 3:00 3:30 25 25 25 25 29 4:00 4:30 5:00 25 Table 3: Heat of Neutralization Setup Data Collected Measurement 27C Temperature of HCl solution, C OO e 3 Chemistry 1 Spring 2022 + Biological Evolution Chemistry 1 Spring 2022 Temperature of NaOH solution, C Concentration of NaOH solution, M 25c 0.9652 Table 4: Time and Temperature, Enthalpy of Acid-Base Reaction 0:05 Time (min: sec) Temperature (C) 0:00 23.c 24 0:10 29 0:15 30 0:20 30 0:25 30 0:30 30 0:35 30 0:40 30 0:45 30 0:50 30 0:55 30 1:00 20 1:30 30 2:00 30 2:30 30 3:00 30 3:30 30 4:00 30 30 5:00 29.8 4:30 Part 1 Data Analysis: Specific heat of metal calculations Calculation for heat gained by water Data Collected Toog 4.1846J/g C 210 Measurement Mass of water in calorimeter, g Specific heat of water Initial temperature of water in calorimeter, C Maximum final temperature of water from graph of data, C Temperature change of water AT = (T final Tinitial) Heat gained by water aw q = mw (4.184 J/g C)*AT 25.0 400 25C - 2you Calculation for specific heat of metal Data Collected Measurement 58 g Mass of metal, g of 24 Initial temperature of metal in calorimeter, C Maximum final temperature of metal from graph of data, C (same as the final temperature of water) Temperature change of metal, AT = (Tfinal - Tinitial) Specific heat of metal: mwcwA TW Cm mm ATM 21.c 25c yu 25-27.c Part II Data Analysis: Enthalpy of Reaction calculations Write the balanced equation for the reaction of HCl with NaOH: Measurement Data Collected 50.0 mL Volume of HCI 50.0 mL Volume of NaOH 100. Om 27-c 25oC 26C Total volume of final mixture Total mass of final mixture (density = 1g/mL) Initial temperature of HCI, C Initial temperature of NaOH, C Average initial temperature, C Exact molar concentration of NaOH, M Maximum final temperature from graph, C Temperature change = (T final - Tinitial) Specific heat of mixture Heat evolved, a 29.8.0 29.8.0 23.0 6.800 AT = 4.184 J/g C 9 = -cmat Moles of OH reacted Moles of H2O formed Heat evolved per mole of H20, AH,, kJ/mol O Data Sheet Table 1: Calorimeter Experimental Setup Data Collected 57.998 > 58.00 1oooc Measurement Mass of metal rod, g Temperature of boiling water bath, C Calorimeter Water temperature, C Water volume, ml Water density, g/mL Calculated mass of water, g 21 c 100.0 m 1 ml/g 00 Table 2: Time and Temperature, Specific Heat of Metal Time (min: sec) Temperature (C) 0:00 21 0:05 23 0:10 23 0:15 25 0:20 25 0:25 25 0:30 25.1 0:35 25 0:40 2 S 0:45 25 0:50 0:55 25 1:00 28 1:30 2:00 25 2:30 25 25 25 3:00 25 3:30 25 25 4:00 4:30 25 25 5:00 Table 3: Heat of Neutralization Setup Data Collected Measurement Temperature of HCl solution, C 27C va T Temperature of NaOH solution, C Concentration of NaOH solution, M 25C 0.9652 0:00 Table 4: Time and Temperature, Enthalpy of Acid-Base Reaction Time (min: sec) Temperature (C) 23-0 0:05 24 0:10 29 0:15 30 0:20 30 0:25 30 0:30 30 0:35 30 0:40 30 0:45 30 0:50 30 0:55 30 1:00 30 1:30 30 2:00 30 2:30 30 3:00 30 3:30 30 4:00 30 4:30 30 5:00 29.8 100 g x 4 1 - 418.1 x 4C =167 Part / Data Analysis: Specific heat of metal calculations Calculation for heat gained by water Measurement Data Collected Mass of water in calorimeter, g 1009 2100 4.1846 J/g C Specific heat of water Initial temperature of water in calorimeter, C Maximum final temperature of water from graph of data, C Temperature change of water AT = (Tfinal Tinitial) Heat gained by water aw q = mw (4.184 J/g C) AT 25C - 2you 25 oC 400 1673.84 J Calculation for specific heat of metal Data Collected Measurement 58 g Mass of metal, g 24 Chemistry 1 Spring 2022 Initial temperature of metal in calorimeter. Maximum final temperature of metal from graph of data,C (same as the final temperature of water) Temperature change of metal, AT = (Trinal - Tinitial Specific heat of metal: mwcwA TW mm ATm 21c 25oc you 25 - 21.0 Part II Data Analysis: Enthalpy of Reaction calculations Write the balanced equation for the reaction of HCI with NaOH: Measurement Data Collected Volume of HCI 50.0 mL 50.0 mL 100. Om 100g 27-cu 2 soc 2. GoC Volume of NaOH Total volume of final mixture Total mass of final mixture (density = 1g/mL) Initial temperature of HCI, C Initial temperature of NaOH, C Average initial temperature, C Exact molar concentration of NaOH, M. Maximum final temperature from graph, C Temperature change AT = (T final - Tinitial) Specific heat of mixture Heat evolved, 29.8.0 29.8' 28-0 66 800 - c 4.184 J/g C q=-CM47 Moles of OH reacted Moles of H2O formed Heat evolved per mole of H20, AH,, kJ/mol
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


