Answered step by step
Verified Expert Solution
Question
1 Approved Answer
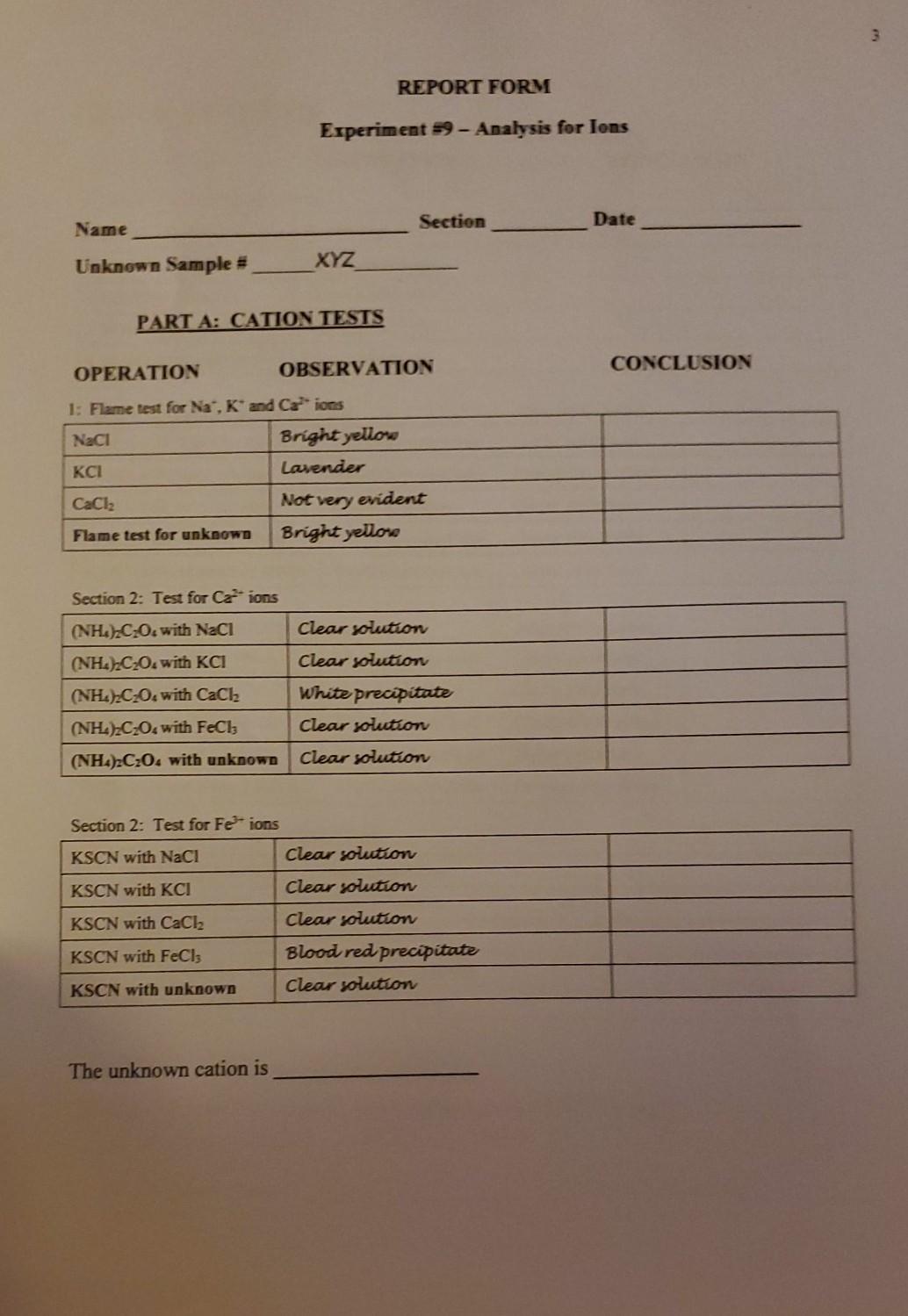
need it asap please thanks REPORT FORM Experiment 59 - Analysis for lons Name Section Date Unknown Sample XYZ PART A: CATION TESTS CONCLUSION OPERATION

need it asap please thanks
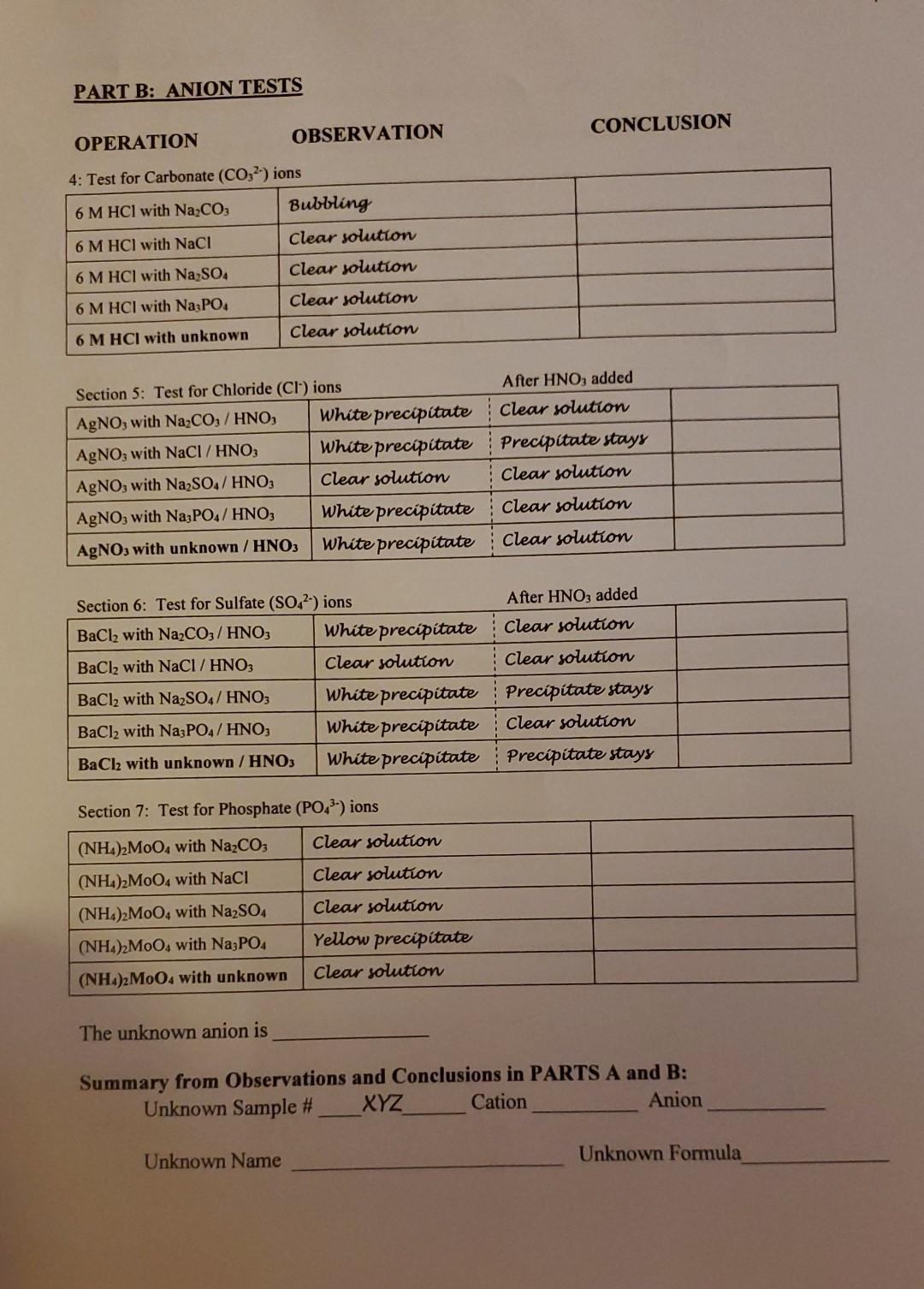
REPORT FORM Experiment 59 - Analysis for lons Name Section Date Unknown Sample XYZ PART A: CATION TESTS CONCLUSION OPERATION OBSERVATION 1: Flame test for Na, K and Ca ions Naci Bright yellona Lavender CaCl2 Not very evident Flame test for unknown Bright yellose Section 2: Test for Ca ions Clear solution (NH).C.O. with NaCl (NH) C20. with KCI (NH4)2CO. with CaCl2 (NH),C20with FeCl, (NH4)2C2O4 with unknown Clear solution White precipitate Clear solution Clear solution Section 2: Test for Fe- ions KSCN with NaCl Clear solution KSCN with KCI Clear solution KSCN with CaCl2 Clear solution KSCN with FeCl, Blood red precipitate KSCN with unknown Clear solution The unknown cation is PART B: ANION TESTS OPERATION OBSERVATION CONCLUSION 4: Test for Carbonate (CO2) ions 6 M HCI with Na2CO: Bubbling 6 M HCl with NaCl Clear solution 6 M HCl with Na SO Clear solution 6 M HCI with Na PO, Clear solution 6 M HCl with unknown Clear solution Section 5: Test for Chloride (CI) ions After HNO3 added AgNO, with NazC0z/HNO, White precipitate Clear solution AgNO; with NaCl / HNO; White precipitate precipitate stays AgNO3 with Na2SO4/ HNO3 Clear solution Clear solution AgNO; with NazPO4/ HNO3 White precipitate Clear solution AgNO, with unknown / HNO3 White precipitate Clear solution After HNO3 added Clear solution Clear solution Section 6: Test for Sulfate (SO42-) ions BaCl2 with Na2CO3 / HNO3 White precipitate BaCl2 with NaCl / HNO3 Clear solution BaCl2 with Na2SO4/ HNO3 White precipitate BaCl2 with Na3PO4/ HNO3 White precipitate BaCl2 with unknown / HNO3 White precipitate Precipitate stays Clear solution Precipitate stays Section 7: Test for Phosphate (PO43-) ions (NH4)2M004 with Na2CO3 Clear solution Clear solution (NH4)2M004 with NaCl (NH4)2M004 with Na2SO4 (NH4)2MoOwith Na3PO4 (NH4)2M004 with unknown Clear solution Yellow precipitate Clear solution The unknown anion is Summary from Observations and Conclusions in PARTS A and B: Unknown Sample # XYZ Cation Anion Unknown Name Unknown Formula 5 Supplemental Questions Answer the following questions and submit them along with your Report Form. 1. Write an equation for the main chemical reaction used to test for each: 9a) CO;2- b) CI c) SO42- 2. Write an equation for the main chemical reaction to test for: a) Ca?+ b) Fe3+ 3. In the space below describe how you tested for Na and K+ ions. 4. A student was given an unknown solid that gave the following test results: A portion of the solution of unknown emitted bubbles of a colorless, odorless gas when hydrochloric acid solution was added. Another portion of the solution gave a blood-red colored solution with potassium thiocyanate solution. a) Which ions are present? Cation Anion b) Name the unknown salt. c) Write the correct formula for the salt. 5. Another student's unknown gave the following test results: a portion of the solution of unknown gave a yellow precipitate with ammonium molybdate reagent. A flame test on some of the solution resulted in a bright yellow flame. a) Which ions are present? Cation Anion b) Name the unknown salt. c) Write the correct formula for the saltStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


