Answered step by step
Verified Expert Solution
Question
1 Approved Answer
sample weight (gms) 2. What osmotic pressure (77) would be developed across the semipermeable membrane of a sample containing: a. 5% sucrose b. 0.9
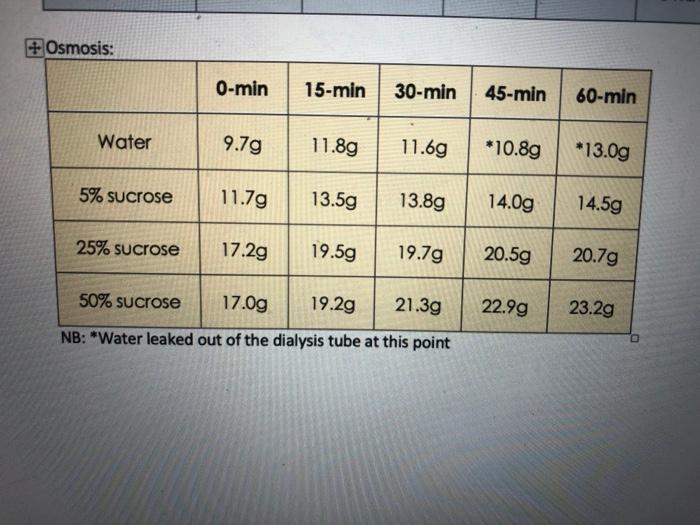
sample weight (gms) 2. What osmotic pressure (77) would be developed across the semipermeable membrane of a sample containing: a. 5% sucrose b. 0.9 % NaCl c. 2 M CaCl 3. a. Is the rate of osmosis constant? Why? GOU 50'.. 1. Results tube # 1. b. What is the relationship between the concentration of sucrose in each bag and the rate of osmosis? The relatenship between the concentration of sucrese in each vay and the rate of osmosis is . Tonicity hct 2 solution tonicity response (lysis, crenation, etc.) +Osmosis: Water 11.6g *13.0g 13.8g 14.0g 14.5g 19.5g 19.7g 20.5g 20.7g 17.0g 19.2g 21.3g NB: "Water leaked out of the dialysis tube at this point 5% sucrose 25% sucrose 0-min 15-min 30-min 45-min 60-min 50% sucrose 9.7g 11.8g 11.7g 13.5g 17.2g *10.8g 22.99 23.2g
Step by Step Solution
★★★★★
3.59 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
iCRT iMRT inrtv where M molarity n moles v vol in litre ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


