Question: Exit gas from a chlorinator consists of a mixture of 20 mol% chlorine in air. This concentration is to be reduced to 1% chlorine by
Exit gas from a chlorinator consists of a mixture of 20 mol% chlorine in air. This concentration is to be reduced to 1% chlorine by water absorption in a packed column to operate isothermally at 20°C and atmospheric pressure. Using the following equilibrium x-y data, calculate for 100 kmol/h of feed gas:
(a) The minimum water rate in kilograms per hour.
(b) NOG for twice the minimum water rate. Data for x-y at 20°C (in chlorine molefractions):
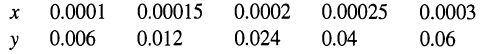
0.0001 0.00025 0.0003 0.0002 0.00015 0.006 0.012 0.024 0.04 0.06 y
Step by Step Solution
3.51 Rating (168 Votes )
There are 3 Steps involved in it
Solve this problem using mole ratios Feed gas is 80 kmolh of air G and 20 kmolh of Cl 2 with Y in 2080 025 Exit gas has Y out 199 00101 X in 00 Highest value of Y in the table of equilibrium data is 0... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (240).docx
120 KBs Word File


