Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Omega 3 Syllabus (?) Help Modules Grades 7 202.0gA2696J4.184((J)/(g))*deg CDelta ^(')deg C->17> oot(0)(0)ASigma phi (Delta T), of the water if 202.0g of water sat
\\\\Omega 3\ Syllabus\ (?) Help\ Modules\ Grades\ 7\
202.0gA2696J4.184((J)/(g))*\\\\deg C\\\\Delta ^(')\\\\deg C->17>\\\ oot(0)(0)A\\\\Sigma \\\\phi (\\\\Delta T), of the water if 202.0g of water sat in the copper pipe from part A,\ Harmonize\ releasing 2696J of energy to the pipe? The specific heat of water is 4.184((J)/(g))*\\\\deg C\ NetTutor\ Express your answer to four significant figures.\ View Available Hint(s)\ \\\\Delta ^(')\ \\\\deg C\ ->1\ Provide Feedback\ (2) 4 of 7>\ iew Available Hint(s)\ \\\ oot(0)(0)\ A\\\\Sigma \\\\phi \ h\ 0\ ? 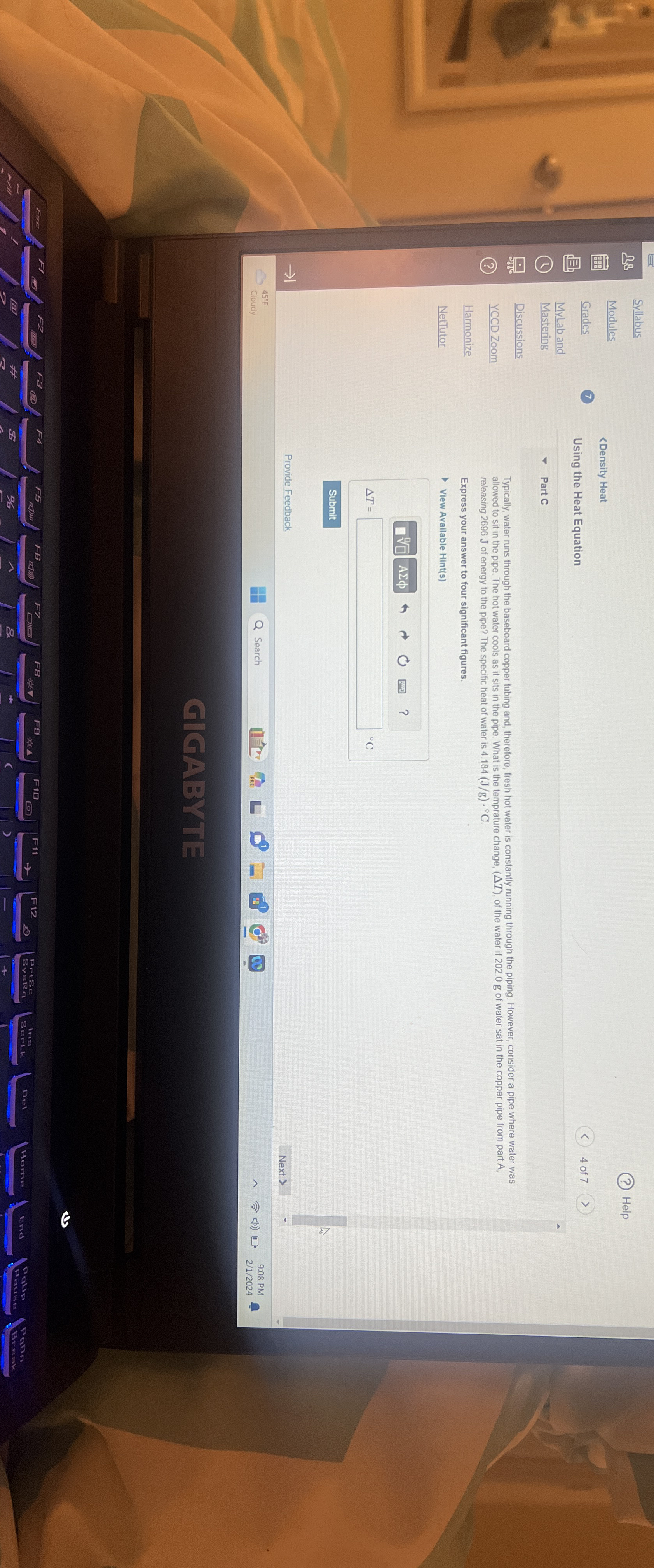
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


