Answered step by step
Verified Expert Solution
Question
1 Approved Answer
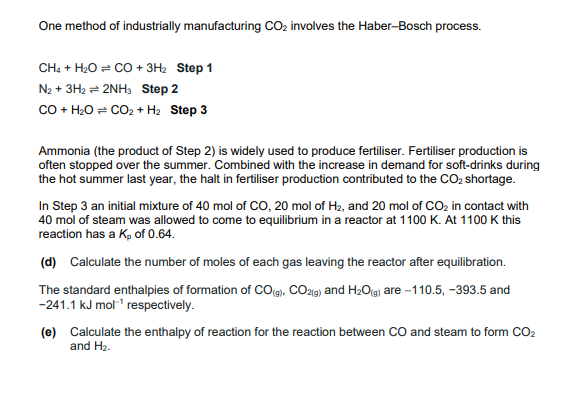
One method of industrially manufacturing C O 2 involves the Haber - Bosch process. C H 4 + H 2 O C O + 3
One method of industrially manufacturing involves the HaberBosch process.
Step
Step
Step
Ammonia the product of Step is widely used to produce fertiliser. Fertiliser production is
often stopped over the summer. Combined with the increase in demand for softdrinks during
the hot summer last year, the halt in fertiliser production contributed to the shortage.
In Step an initial mixture of mol of mol of and mol of in contact with
mol of steam was allowed to come to equilibrium in a reactor at At this
reaction has a of
d Calculate the number of moles of each gas leaving the reactor after equilibration.
The standard enthalpies of formation of and are and
respectively.
e Calculate the enthalpy of reaction for the reaction between and steam to form
and
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


