Question
One mole of solid silver superheated to 1000 C is allowed to melt at the same temperature. Use the data given below to calculate
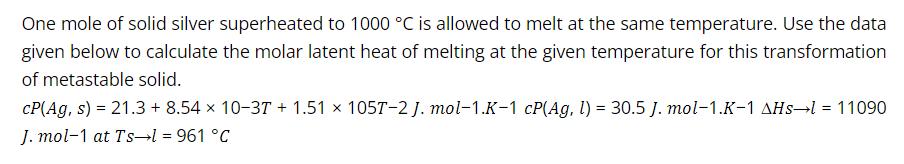
One mole of solid silver superheated to 1000 C is allowed to melt at the same temperature. Use the data given below to calculate the molar latent heat of melting at the given temperature for this transformation of metastable solid. CP(Ag, s) = 21.3 + 8.54 x 10-37 + 1.51 x 105T-2 J. mol-1.K-1 cP(Ag, l) = 30.5 J. mol-1.K-1 AHs-l = 11090 J. mol-1 at Ts l = 961 C
Step by Step Solution
3.32 Rating (164 Votes )
There are 3 Steps involved in it
Step: 1
Date 1 20 Page No Guien Molei of Solid delver I mod Meltingpaint of Silber 961C Heat o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Managerial Accounting
Authors: Ray H. Garrison, Eric W. Noreen, Peter C. Brewer
13th Edition
978-0073379616, 73379611, 978-0697789938
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



