Answered step by step
Verified Expert Solution
Question
1 Approved Answer
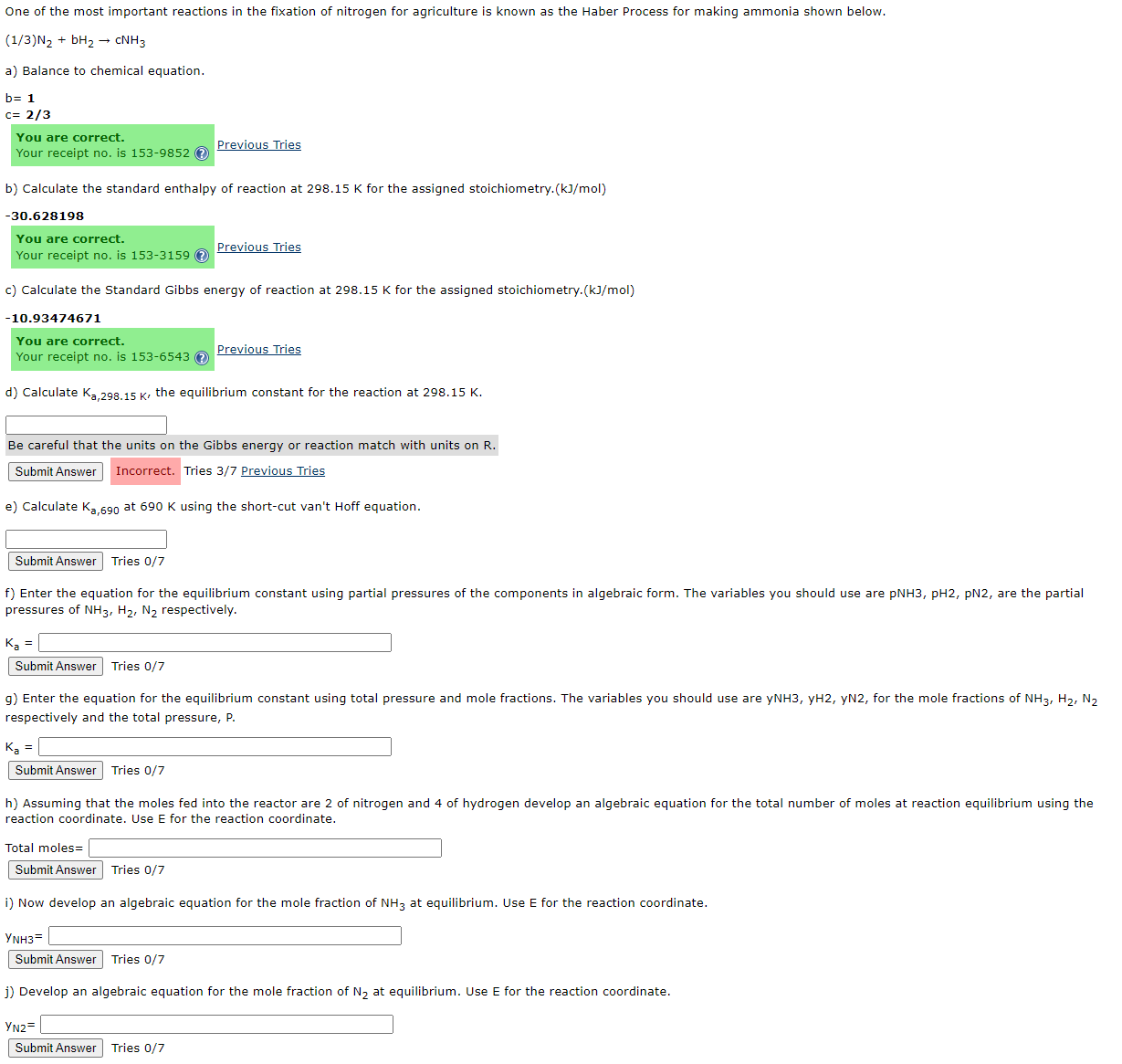
One of the most important reactions in the fixation of nitrogen for agriculture is known as the Haber Process for making ammonia shown below. (
One of the most important reactions in the fixation of nitrogen for agriculture is known as the Haber Process for making ammonia shown below.
a Balance to chemical equation.
You are correct.
Your receipt no is
Previous Tries
b Calculate the standard enthalpy of reaction at for the assigned stoichiometry.
You are correct.
Your receipt no is Previous Tries
c Calculate the Standard Gibbs energy of reaction at for the assigned stoichiometry.
You are correct.
Your receipt no is Previous Tries
Be careful that the units on the Gibbs energy or reaction match with units on
Tries Previous Tries
e Calculate at using the shortcut van't Hoff equation.
Submit Answer Tries
f Enter the equation for the equilibrium constant using partial pressures of the components in algebraic form. The variables you should use are pNH pH pN are the partial
pressures of respectively.
Submit Answer Tries
g Enter the equation for the equilibrium constant using total pressure and mole fractions. The variables you should use are for the mole fractions of
respectively and the total pressure,
Tries
h Assuming that the moles fed into the reactor are of nitrogen and of hydrogen develop an algebraic equation for the total number of moles at reaction equilibrium using the
reaction coordinate. Use E for the reaction coordinate.
Total moles
Tries
i Now develop an algebraic equation for the mole fraction of at equilibrium. Use for the reaction coordinate.
Tries
j Develop an algebraic equation for the mole fraction of at equilibrium. Use for the reaction coordinate.
Tries
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


