Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Water-treatment processes that generate hydroxyl radicals are often used to remove organic chemicals that contaminate groundwater. The hydroxyl radical (OH) is a very reactive
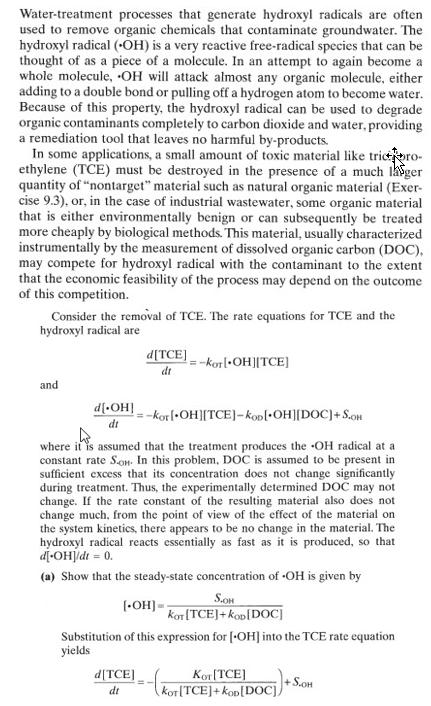
Water-treatment processes that generate hydroxyl radicals are often used to remove organic chemicals that contaminate groundwater. The hydroxyl radical (OH) is a very reactive free-radical species that can be thought of as a piece of a molecule. In an attempt to again become a whole molecule, OH will attack almost any organic molecule, either adding to a double bond or pulling off a hydrogen atom to become water. Because of this property, the hydroxyl radical can be used to degrade organic contaminants completely to carbon dioxide and water, providing a remediation tool that leaves no harmful by-products. In some applications, a small amount of toxic material like tricro- ethylene (TCE) must be destroyed in the presence of a much ger quantity of "nontarget" material such as natural organic material (Exer- cise 9.3), or, in the case of industrial wastewater, some organic material that is either environmentally benign or can subsequently be treated more cheaply by biological methods. This material, usually characterized instrumentally by the measurement of dissolved organic carbon (DOC), may compete for hydroxyl radical with the contaminant to the extent that the economic feasibility of the process may depend on the outcome of this competition. Consider the removal of TCE. The rate equations for TCE and the hydroxyl radical are d[TCE] dt =-kor[OH][TCE] and d[OH] dt =-kor[OH][TCE]-kop[-OH][DOC]+S.OH where it is assumed that the treatment produces the "OH radical at a constant rate Son. In this problem, DOC is assumed to be present in sufficient excess that its concentration does not change significantly during treatment. Thus, the experimentally determined DOC may not change. If the rate constant of the resulting material also does not change much, from the point of view of the effect of the material on the system kinetics, there appears to be no change in the material. The hydroxyl radical reacts essentially as fast as it is produced, so that d[-OH]/dt = 0. (a) Show that the steady-state concentration of "OH is given by [OH]-- S.OH Kor[TCE]+Kop[DOC] Substitution of this expression for [OH] into the TCE rate equation yields d[TCE] Kor[TCE] S.OH dt kor TCE]+Kop[DOC])
Step by Step Solution
★★★★★
3.33 Rating (153 Votes )
There are 3 Steps involved in it
Step: 1
Solo a To Find the steadystate concentration of OH set dxon to zero dt se...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


