Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Organic esters are an important class of chemicals. The most common way to produce them is the acid catalyzed reaction between carboxylic acids and
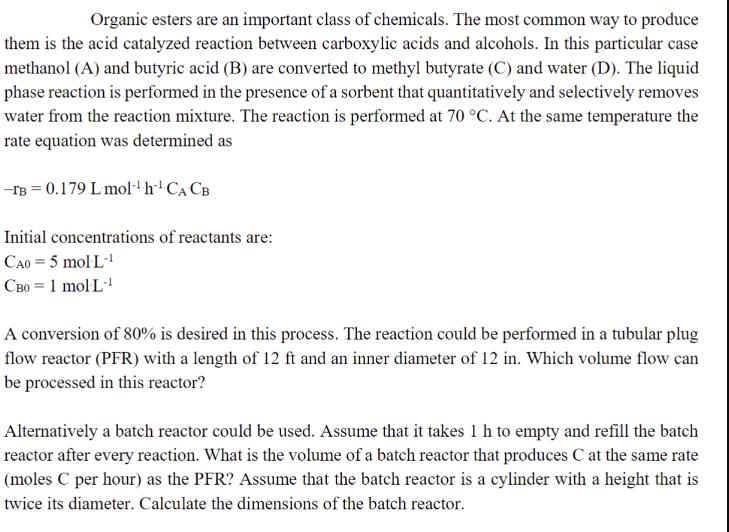
Organic esters are an important class of chemicals. The most common way to produce them is the acid catalyzed reaction between carboxylic acids and alcohols. In this particular case methanol (A) and butyric acid (B) are converted to methyl butyrate (C) and water (D). The liquid phase reaction is performed in the presence of a sorbent that quantitatively and selectively removes water from the reaction mixture. The reaction is performed at 70 C. At the same temperature the rate equation was determined as -TB 0.179 L mol h CA CB Initial concentrations of reactants are: CAO = 5 mol L-1 CB0 = 1 molL-1 A conversion of 80% is desired in this process. The reaction could be performed in a tubular plug flow reactor (PFR) with a length of 12 ft and an inner diameter of 12 in. Which volume flow can be processed in this reactor? Alternatively a batch reactor could be used. Assume that it takes 1 h to empty and refill the batch reactor after every reaction. What is the volume of a batch reactor that produces C at the same rate (moles C per hour) as the PFR? Assume that the batch reactor is a cylinder with a height that is twice its diameter. Calculate the dimensions of the batch reactor.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


