Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Oxygen dissolves into and reacts irreversibly with aqueous sodium sulphite solutions contained in a vertical container of height H. If the gas solubility is denoted
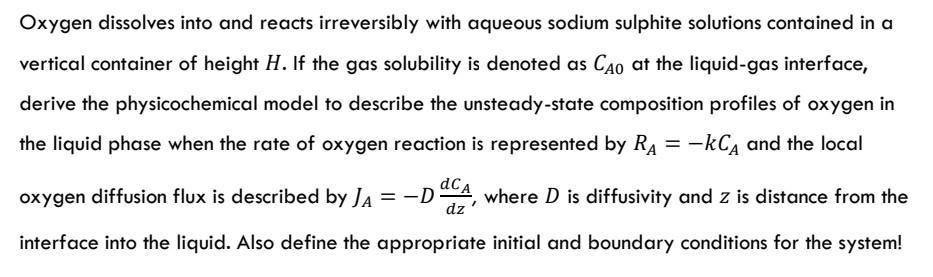
Oxygen dissolves into and reacts irreversibly with aqueous sodium sulphite solutions contained in a vertical container of height H. If the gas solubility is denoted as Cao at the liquid-gas interface, derive the physicochemical model to describe the unsteady-state composition profiles of oxygen in the liquid phase when the rate of oxygen reaction is represented by RA = -kCA and the local oxygen diffusion flux is described by JA =-DOCA, where D is diffusivity and Z is distance from the D z dz interface into the liquid. Also define the appropriate initial and boundary conditions for the system
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


