Answered step by step
Verified Expert Solution
Question
1 Approved Answer
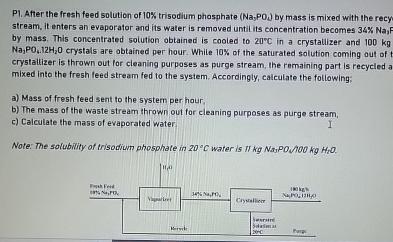
P 1 . After the fresh feed solution of 1 0 W trisodium phasphate ( N a 3 P O ) by mass is mixed
P After the fresh feed solution of trisodium phasphate by mass is mixed with the recy stream, it enters an evaporator and its water is removed untilits concentration becomes by mass. This concentrated solution obtained is cooled to in a crystallizer and crystals are obtained per hour. While of the saturated solution coming out of crystallizer is thrown out lor cleaning purposes as purge stream, the remaining part is recycled a mixed into the fresh leed stream fed to the system. Accordingly, calculate the following:
a Mass of fresh feed sent to the system per haur,
b The mass of the waste stream thrown out for cleaning purposes as purge stream,
c Calculate the mass of evaporated water
I
Note: The solubwity of trisodium phosphate in water is MOO
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


