Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A Acetylene, C_(2)H_(2) , can be converted to ethane, C_(2)H_(6) , by a process known as hydrogenation. The reaction is C_(2)H_(2)(g)+2H_(2)(g)C_(2)H_(6)(g) Given the
Part A\ Acetylene,
C_(2)H_(2), can be converted to ethane,
C_(2)H_(6), by a process known as hydrogenation. The reaction is\
C_(2)H_(2)(g)+2H_(2)(g)C_(2)H_(6)(g)\ Given the following data at standard conditions (all pressures equal to
1atmand the common reference temperature
298K), what is the value of
K_(p)fo reaction?\ \\\\table[[Substance,\\\\table[[
\\\\Delta G_(f)\\\\deg 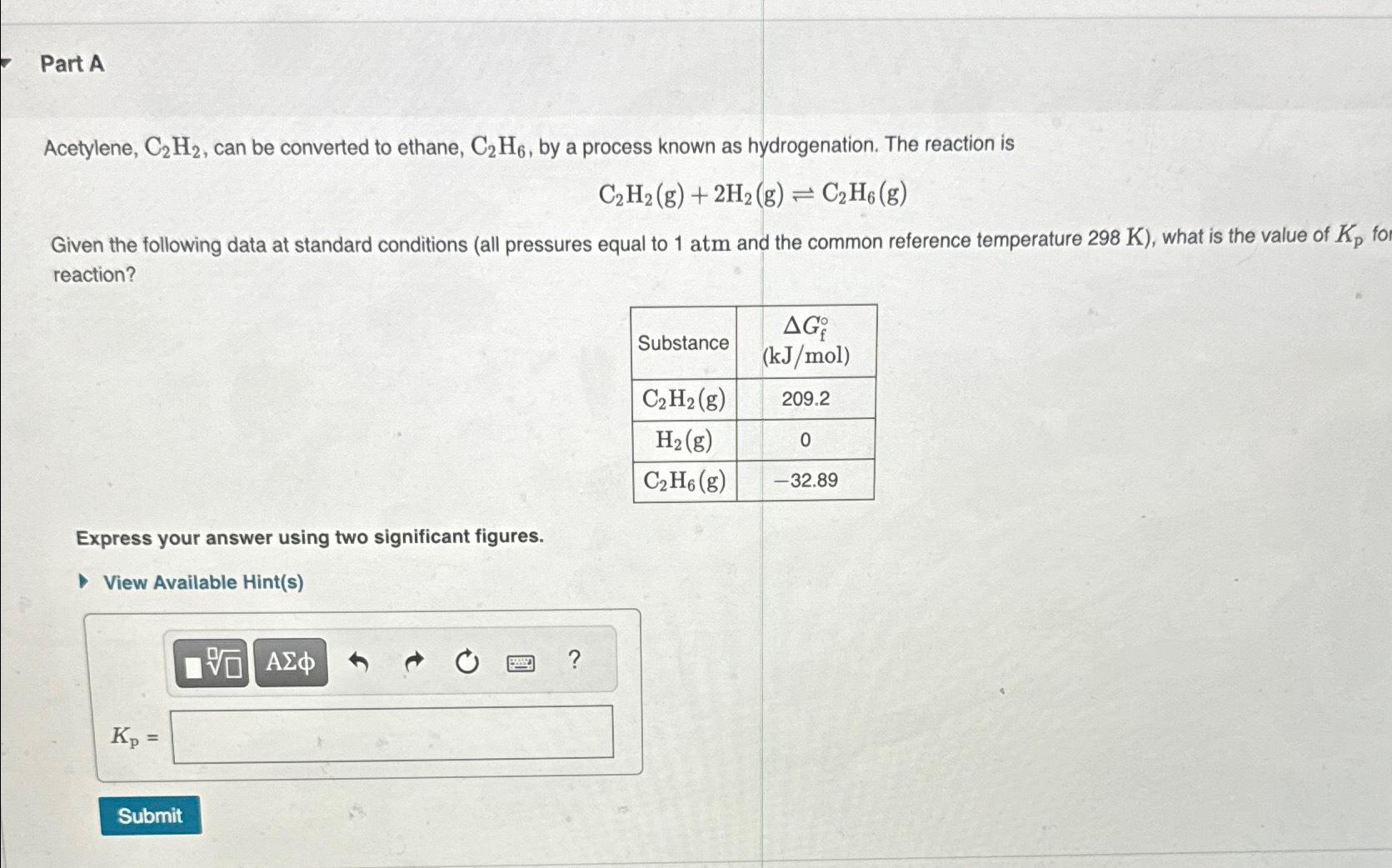
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


