Question
+ Enthalpy of Reaction: State and Stoichiometry Use the data below to answer the questions. Substance AH (kJ/mol) C(g) CF.(g) CH(g) 718.4 -679.9 -74.8
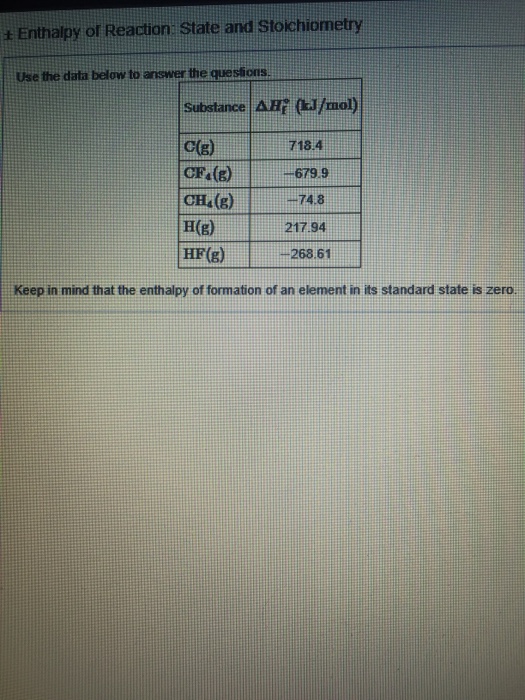

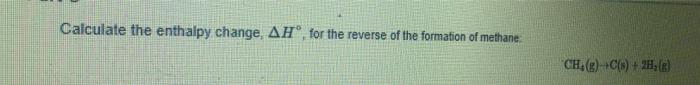
+ Enthalpy of Reaction: State and Stoichiometry Use the data below to answer the questions. Substance AH (kJ/mol) C(g) CF.(g) CH(g) 718.4 -679.9 -74.8 217.94 -268.61 H(g) HF(g) Keep in mind that the enthalpy of formation of an element in its standard state is zero. Calculate the enthalpy change, AH, for the "expansion" of methane; CH(g) C(g) + 4H(g) Calculate the enthalpy change, AH, for the reverse of the formation of methane CH(g) +C(s) + 2H(g)
Step by Step Solution
3.43 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Fundamentals of Investments Valuation and Management
Authors: Bradford D. Jordan, Thomas W. Miller
5th edition
978-007728329, 9780073382357, 0077283295, 73382353, 978-0077283292
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



