Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A Consider a monatomic gas of particles each with mass m . What is v x , r m s = ? 2 ,
Part A
Consider a monatomic gas of particles each with mass What is the root mean square rms of the component of velocity of the gas particles if the gas is at an absolute temperature
Express your answer in terms of and other given quantities.
View Available Hints
Part B
Now consider the same system: a monatomic gas of particles of mass except in three dimensions. Find the rms speed if the gas is at an absolute temperature
Express your answer in terms of and other given quantities.
Part C
What is the rms speed of molecules in air at Air is composed mostly of molecules, so you may assume that it has molecules of average mass
Express your answer in meters per
Now consider a rigid dumbbell with two masses, each of mass spaced a distance apart.
Part D
Find the rms angular speed of the dumbbell about a single axis taken to be the axis assuming that the dumbbell is lined up on the axis and is in equilibrium at temperature
Express the rms angular speed in terms of and other given quantities.
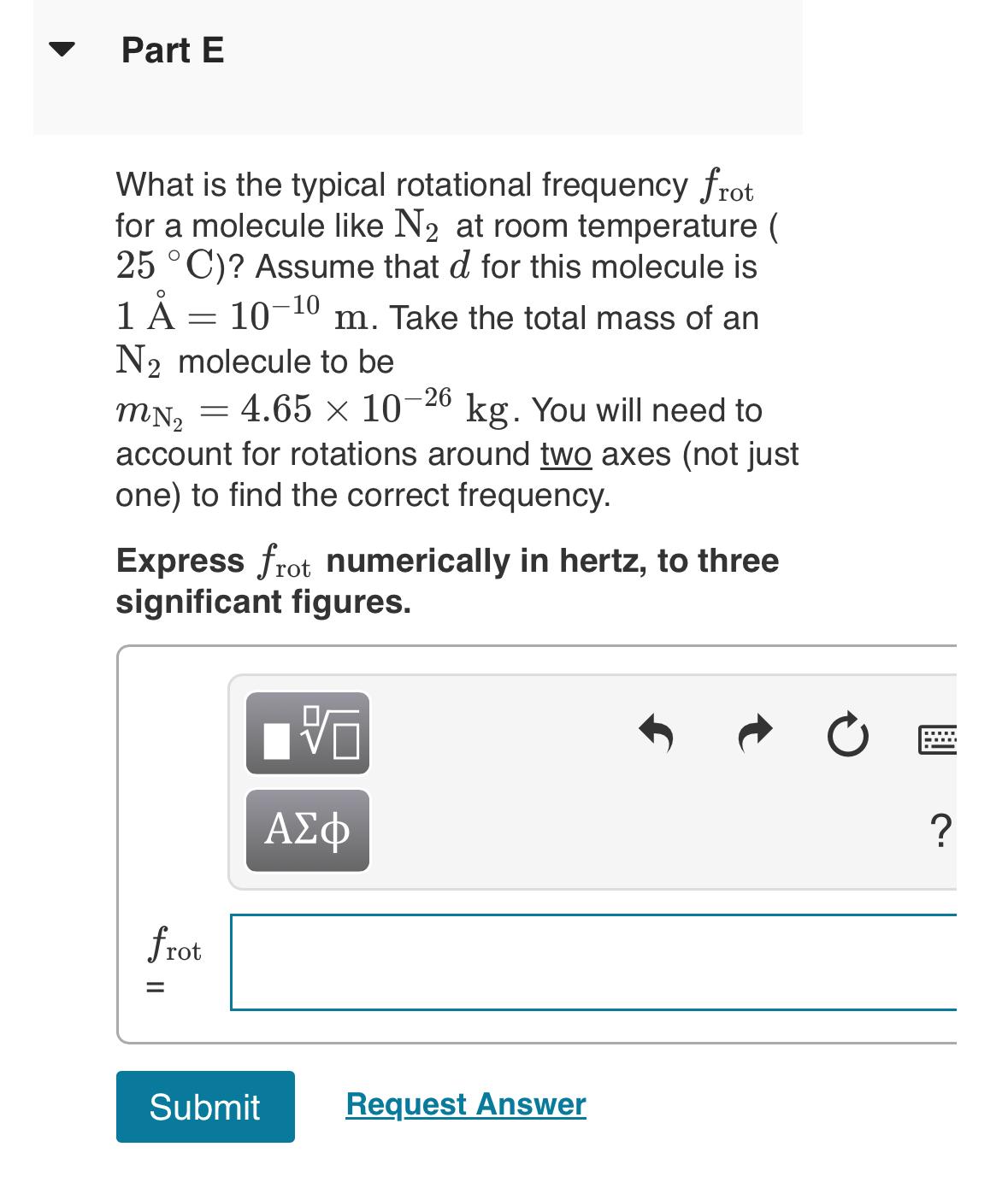
Part E
What is the typical rotational frequency for a molecule like at room temperature Assume that for this molecule is Take the total mass of an molecule to be You will need to account for rotations around two axes not just one to find the correct frequency.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


