Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A Draw the Lewis structure of PH3. button before clicking on the molecule. To add bonds connect atoms with a line To add
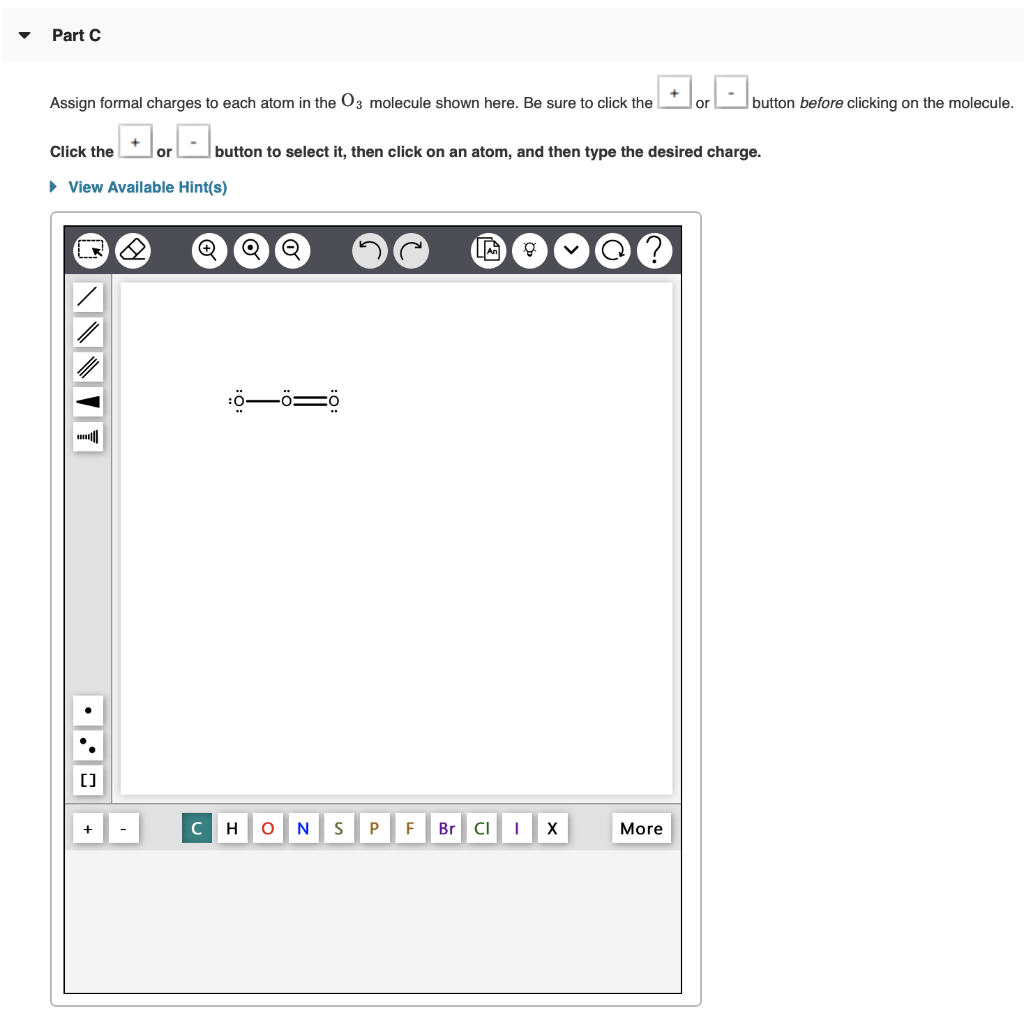
Part A Draw the Lewis structure of PH3. button before clicking on the molecule. To add bonds connect atoms with a line To add lone pairs, click the Draw the molecule by placing atoms on the grid and connecting them with bonds. Include lone pairs of electrons and hydrogen atoms. View Available Hint(s) + [] 2 Q H QQ O N S P LA F Br CI g I X More Part B Draw the Lewis structure of NO. To add lone pairs, click the Q& Draw the molecule by placing atoms on the grid and connecting them with bonds. Include all lone pairs of electrons. Use square brackets to denote the overall charge. View Available Hint(s) NNNIY [] '. . button before clicking on the molecule. To add bonds connect atoms with a line Q Q Q H ONS P F Br CII X More Part C Assign formal charges to each atom in the 03 molecule shown here. Be sure to click the or Click the + [] or View Available Hint(s) button to select it, then click on an atom, and then type the desired charge. Q :00=0 C H O N S P F Br Cl g X button before clicking on the molecule. More Part D Based on formal charges, draw the most preferred Lewis structure for the chlorate ion, C1O. To add lone pairs, click the button before clicking on the molecule. To add bonds connect atoms with a line. To add formal charges, click theorbutton before clicking on the molecule. Draw the molecule by placing atoms on the grid and connecting them with bonds. Include lone pairs of electrons. Use square brackets to denote the overall charge. View Available Hint(s) NNNII [] Q QTQ HO N S P F Br More
Step by Step Solution
★★★★★
3.44 Rating (176 Votes )
There are 3 Steps involved in it
Step: 1
Lewis structure of PHz 2 Lewis structure of NO DOND V 3 O o H 0 2 0 3 0 4 0 0 3 Formal ch...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


