Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A The rate of the reaction in terms of the disappearance of reactant includes the change in the concentration of the reactant, the time
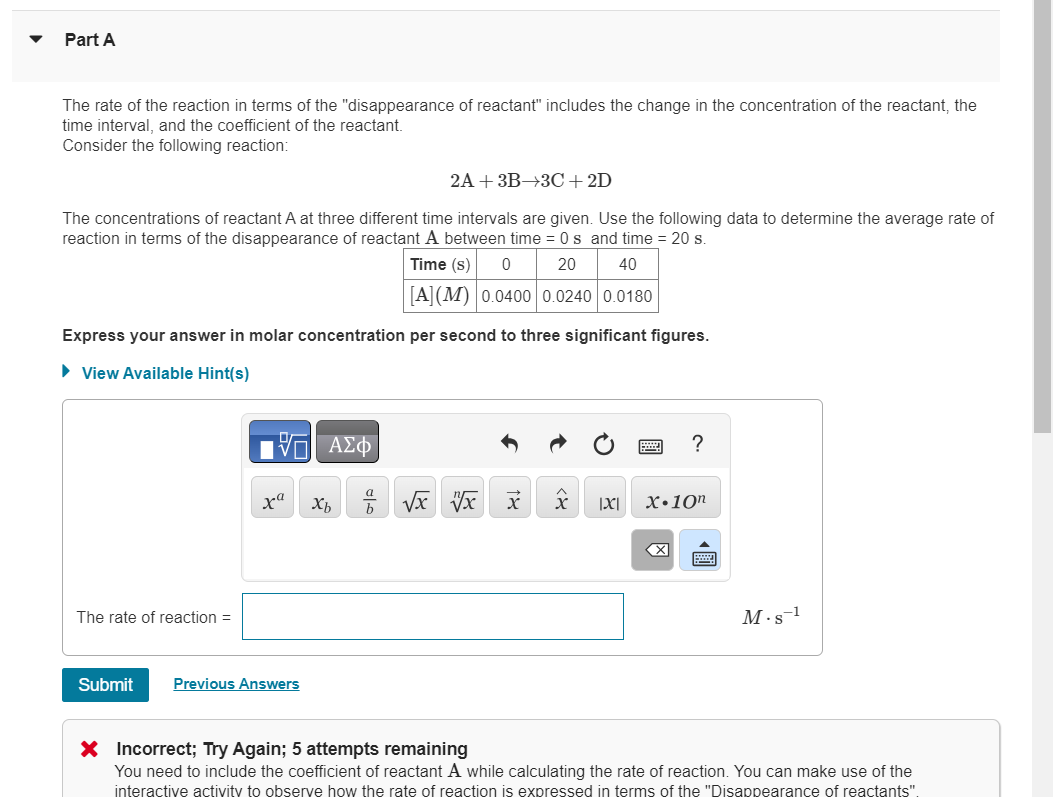
Part A
The rate of the reaction in terms of the "disappearance of reactant" includes the change in the concentration of the reactant, the
time interval, and the coefficient of the reactant.
Consider the following reaction:
The concentrations of reactant A at three different time intervals are given. Use the following data to determine the average rate of
reaction in terms of the disappearance of reactant A between time and time
Express your answer in molar concentration per second to three significant figures.
View Available Hints
The rate of reaction
X Incorrect; Try Again; attempts remaining
You need to include the coefficient of reactant A while calculating the rate of reaction. You can make use of the
interactive activity to observe how the rate of reaction is expressed in terms of the "Disanpearance of reactants"
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


