Answered step by step
Verified Expert Solution
Question
1 Approved Answer
= = Patm = 105 Pa A perfectly insulated, frictionless piston- cylinder device contains air (c = 20 J/mol-K) initially at a temperature of
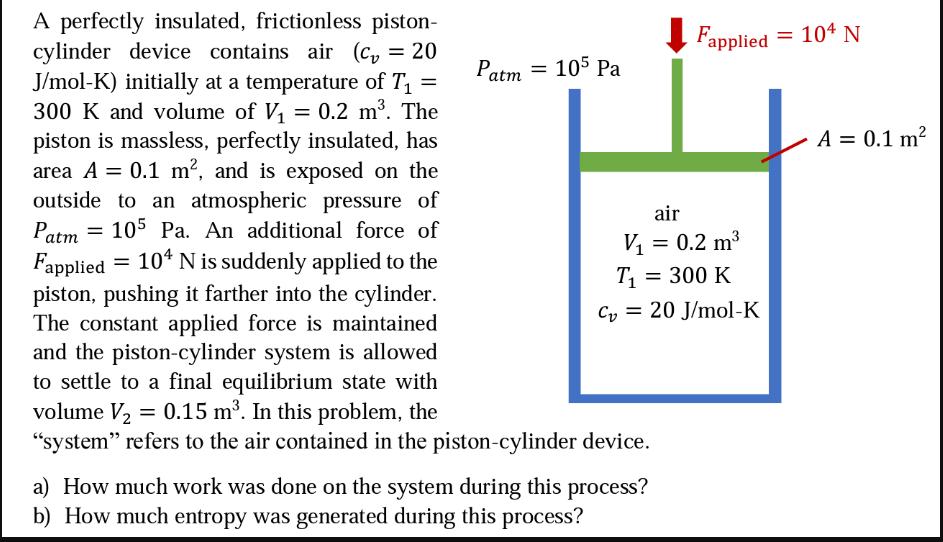
= = Patm = 105 Pa A perfectly insulated, frictionless piston- cylinder device contains air (c = 20 J/mol-K) initially at a temperature of T 300 K and volume of V = 0.2 m. The piston is massless, perfectly insulated, has area A = 0.1 m, and is exposed on the outside to an atmospheric pressure of Patm 105 Pa. An additional force of Fapplied 104 N is suddenly applied to the piston, pushing it farther into the cylinder. The constant applied force is maintained and the piston-cylinder system is allowed to settle to a final equilibrium state with volume V = 0.15 m. In this problem, the "system" refers to the air contained in the piston-cylinder device. = air Fapplied = 104 N V = 0.2 m T = 300 K C20 J/mol-K a) How much work was done on the system during this process? b) How much entropy was generated during this process? A = 0.1 m
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Therefore ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


