A heat engine takes 0.350 mol of a diatomic ideal gas around the cycle shown in the pV-diagram of the figure (Figure 1). Process
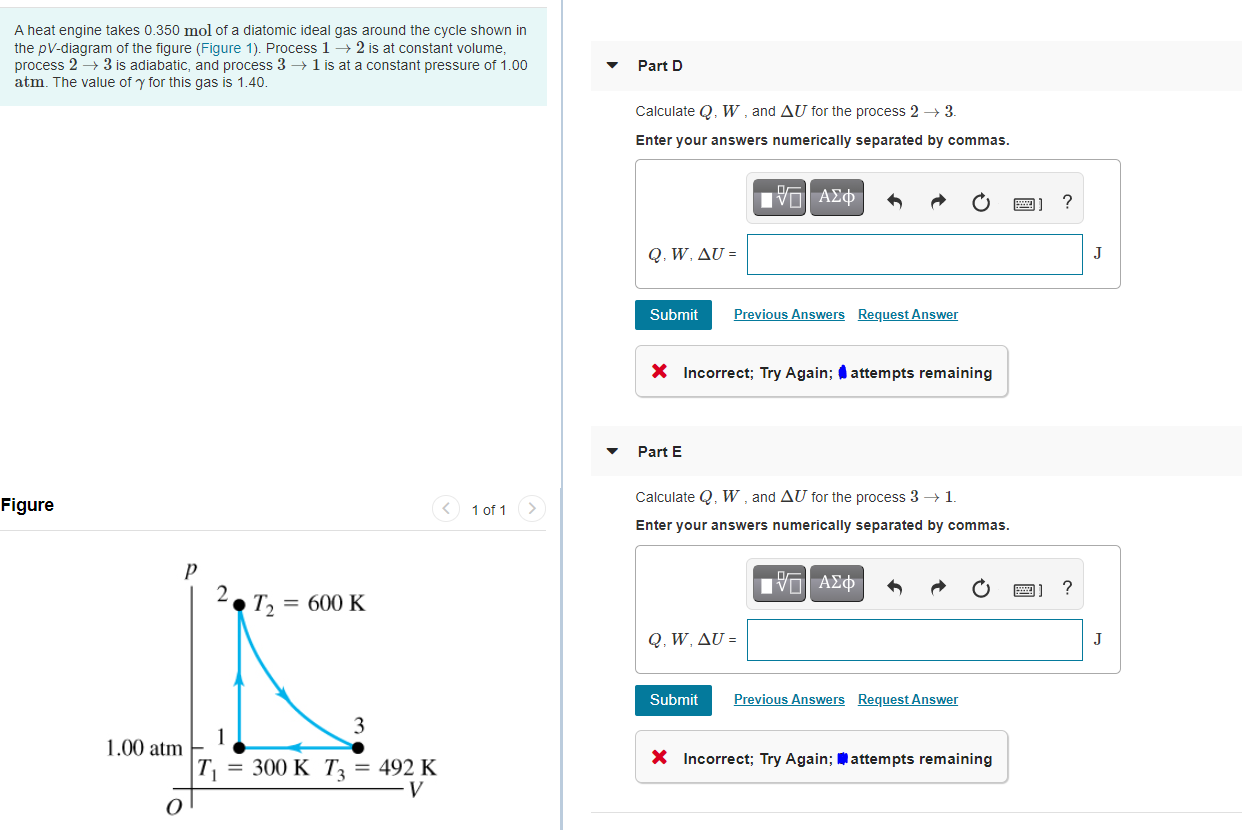
A heat engine takes 0.350 mol of a diatomic ideal gas around the cycle shown in the pV-diagram of the figure (Figure 1). Process 1 2 is at constant volume, process 2 3 is adiabatic, and process 3 1 is at a constant pressure of 1.00 atm. The value of y for this gas is 1.40. Part D Calculate Q. W, and AU for the process 2 3. Enter your answers numerically separated by commas. ? Q. W. U = J Submit Previous Answers Request Answer X Incorrect; Try Again; attempts remaining Part E Calculate Q, W , and AU for the process 3 1. Figure 1 of 1 Enter your answers numerically separated by commas. ? 2. T2 = 600 K Q, W. U - J Submit Previous Answers Request Answer 3 1 1.00 atm X Incorrect; Try Again; attempts remaining T = 300 K T; = 492 K
Step by Step Solution
3.40 Rating (163 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


