Question: Mass of one atom of X is 2.66 x 10-23g, then its 32 g is equal to 32 1) 2) 2.66 x 10-23 x
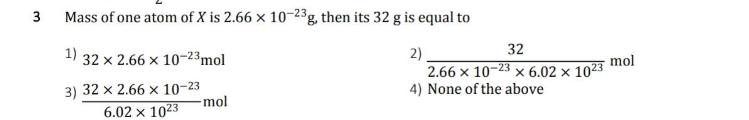
Mass of one atom of X is 2.66 x 10-23g, then its 32 g is equal to 32 1) 2) 2.66 x 10-23 x 6.02 x 1023 4) None of the above 32 x 2.66 x 10-23mol mol 3) 32 x 2.66 x 10-23 -mol 6.02 x 1023 3.
Step by Step Solution
3.33 Rating (153 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


