Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all 4 its all one questions different parts Consider the reaction: SO2(g)+H2O(I)H2SO3(g) Given an initial mass of 19.01gSO2, an excess of H2O, and
please answer all 4 its all one questions different parts 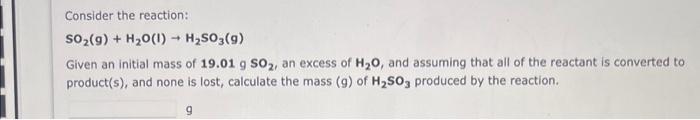



Consider the reaction: SO2(g)+H2O(I)H2SO3(g) Given an initial mass of 19.01gSO2, an excess of H2O, and assuming that all of the reactant is converted to product(s), and none is lost, calculate the mass (g) of H2SO3 produced by the reaction. 9 According to the following reaction, how many grams of water are necessary to form 0.272 moles carbon dioxide? carbon monoxide (g)+ water (I) carbon dioxide (g)+ hydrogen (g) grams water The reaction between potassium chlorate and red phosphorus takes place when you strike a match on a matchbox. If you were to react 62.5g of potassium chlorate ( KClO3 ) with excess red phosphorus, what mass of tetraphosphorus decaoxide ( P4O10) could be produced? KClO3(s)+P4(s)P4O10(t)+KCl(t)(unbalanced)Mass=9 According to the following reaction, how many moles of mercury will be formed upon the complete reaction of 31.6 grams of mercury(II) oxide? mercury(II) oxide (s) mercury (1)+ oxygen (9) moles mercury 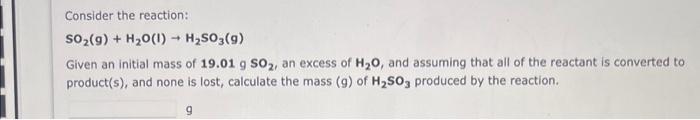



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


