Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer all, i wil upvote. begin{tabular}{|l|l|l|} hline Class of compound & Effect on H+(protons) in solution & multicolumn{1}{|c|}{ Effect on solution pH} hline
please answer all, i wil upvote. 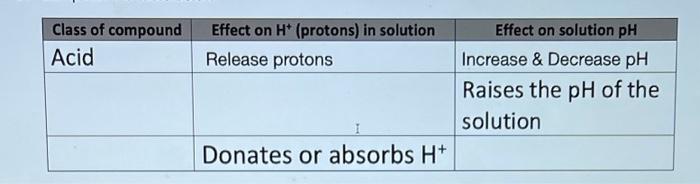
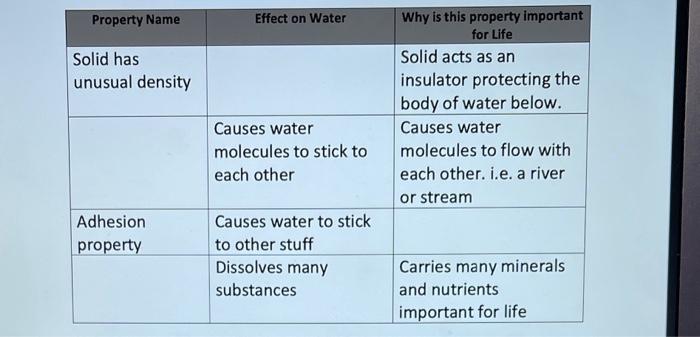

\begin{tabular}{|l|l|l|} \hline Class of compound & Effect on H+(protons) in solution & \multicolumn{1}{|c|}{ Effect on solution pH} \\ \hline Acid & Release protons & Increase \& Decrease pH \\ \hline & & Raises the pH of the solution \\ \hline & Donates or absorbs H+ & \\ \hline \end{tabular} Takes the addition (or Protects life from subtraction) of a lot of temperature energy to change the fluctuations in the physical state of water environment. (solid, liquid, and gas) \begin{tabular}{|l|l|l|} \hline Class of compound & Effect on H+(protons) in solution & \multicolumn{1}{|c|}{ Effect on solution pH} \\ \hline Acid & Release protons & Increase \& Decrease pH \\ \hline & & Raises the pH of the solution \\ \hline & Donates or absorbs H+ & \\ \hline \end{tabular} Takes the addition (or Protects life from subtraction) of a lot of temperature energy to change the fluctuations in the physical state of water environment. (solid, liquid, and gas) 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


