Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer all parts and show all calculation! Follow the standard energy balance steps to determine the water condensed and the heat removed from a
Please answer all parts and show all calculation!
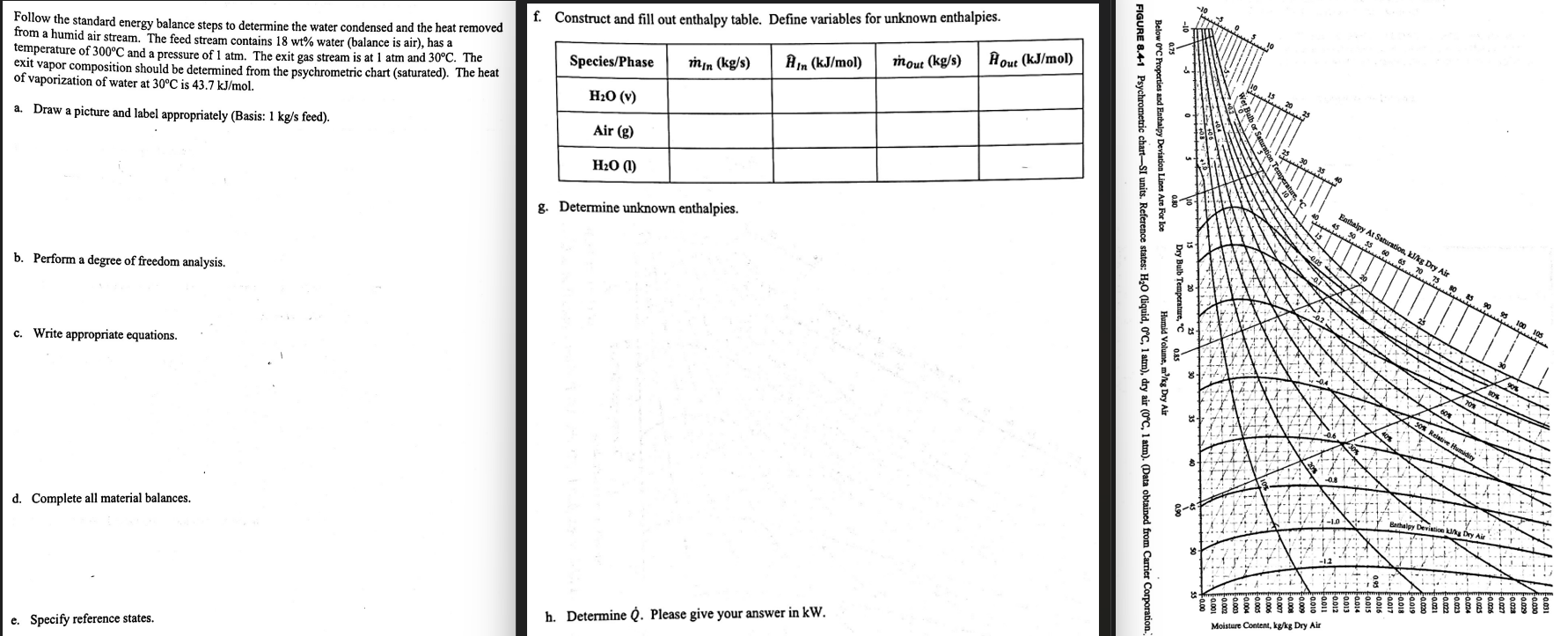
Follow the standard energy balance steps to determine the water condensed and the heat removed
from a humid air stream. The feed stream contains water balance is air has a
temperature of and a pressure of atm. The exit gas stream is at atm and The
exit vapor composition should be determined from the psychrometric chart saturated The heat
of vaporization of water at is
a Draw a picture and label appropriately Basis: feed
b Perform a degree of freedom analysis.
c Write appropriate equations.
d Complete all material balances.
e Specify reference states.
f Construct and fill out enthalpy table. Define variables for unknown enthalpies.
g Determine unknown enthalpies.
h Determine Please give your answer in
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


