Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer all three and I will give you a positive rating! A mixture of 6.00 gram-moles of benzene and 4.00 gram-moles of toluene are

Please answer all three and I will give you a positive rating!
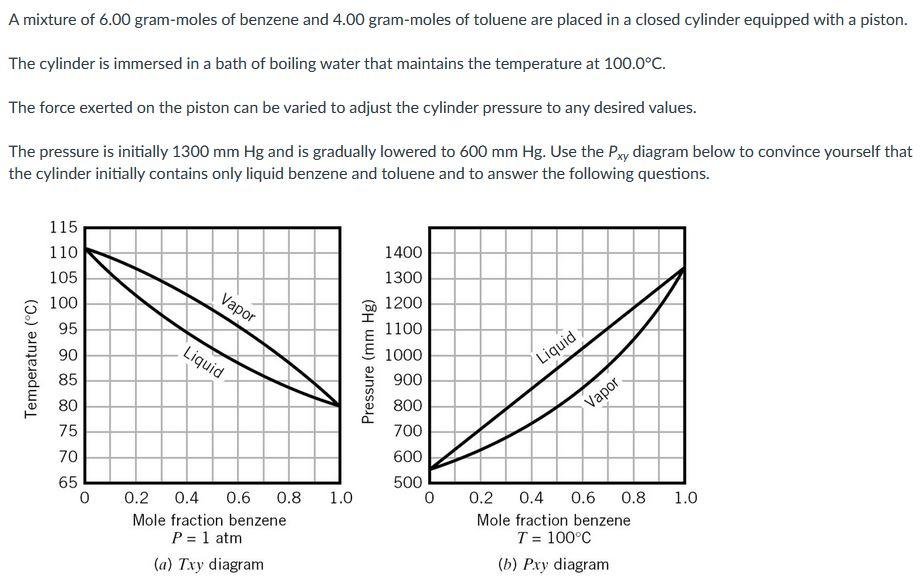
A mixture of 6.00 gram-moles of benzene and 4.00 gram-moles of toluene are placed in a closed cylinder equipped with a piston. The cylinder is immersed in a bath of boiling water that maintains the temperature at 100.0C. The force exerted on the piston can be varied to adjust the cylinder pressure to any desired values. The pressure is initially 1300 mm Hg and is gradually lowered to 600 mm Hg. Use the Pxy diagram below to convince yourself that the cylinder initially contains only liquid benzene and toluene and to answer the following questions. 115 110 105 100 95 Vapor Temperature (C) Liquid 90 85 80 Pressure (mm Hg) 1400 1300 1200 1100 1000 900 800 700 600 Liquid Vapor 75 70 65 0 500 1.0 0 1.0 0.2 0.4 0.6 0.8 Mole fraction benzene P=1 atm (a) Txy diagram 0.2 0.4 0.6 0.8 Mole fraction benzene T = 100C (b) Pxy diagram Estimate the volume of the cylinder contents when the pressure is 1300 mm Hg, 950 mm of Hg, and 600 mm Hg. 1300 mm Hg: i L 950mm of Hg: i L. 600 mm Hg: i LStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


