Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer as much as possible starting from the top. Please. A liquid stream containing three hydrocarbons is fed to a separation train that separates

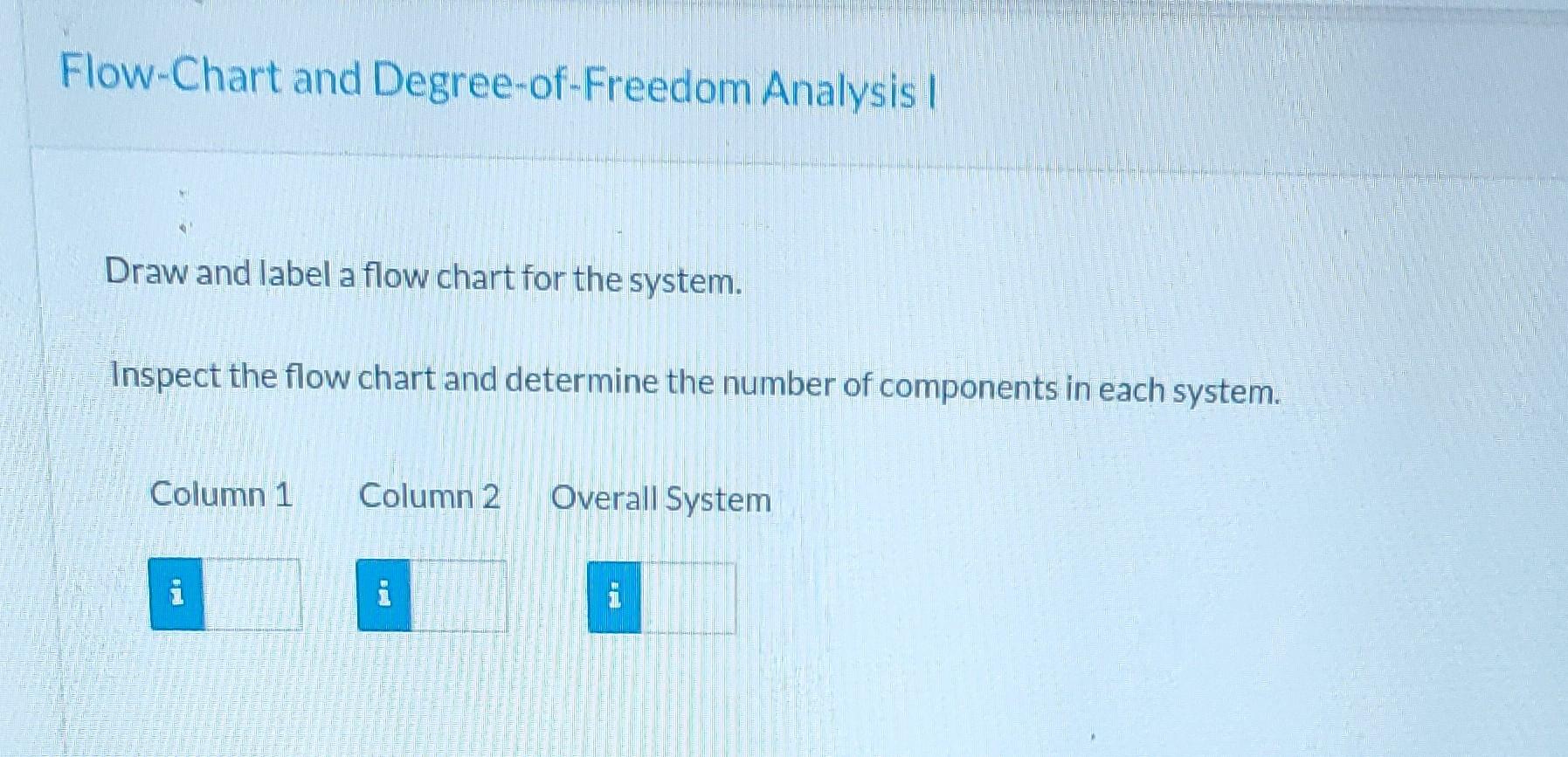
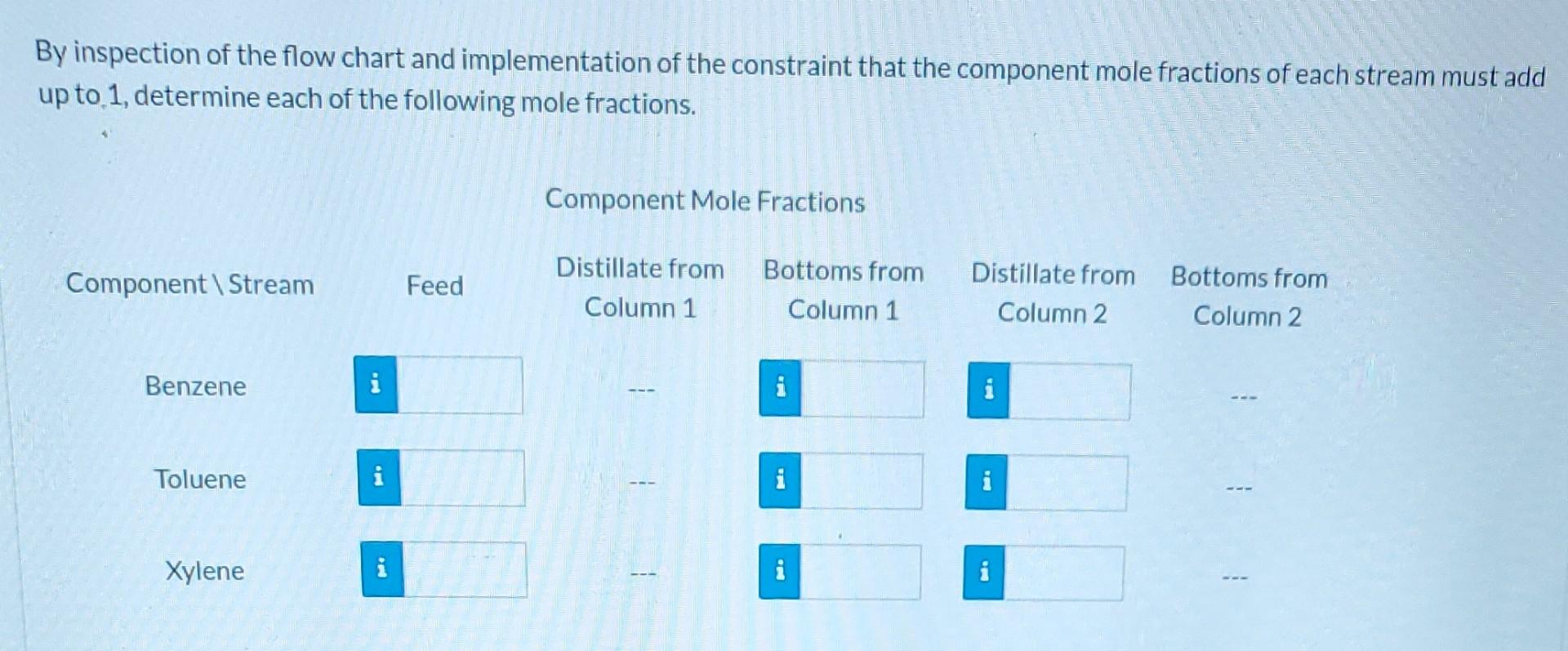
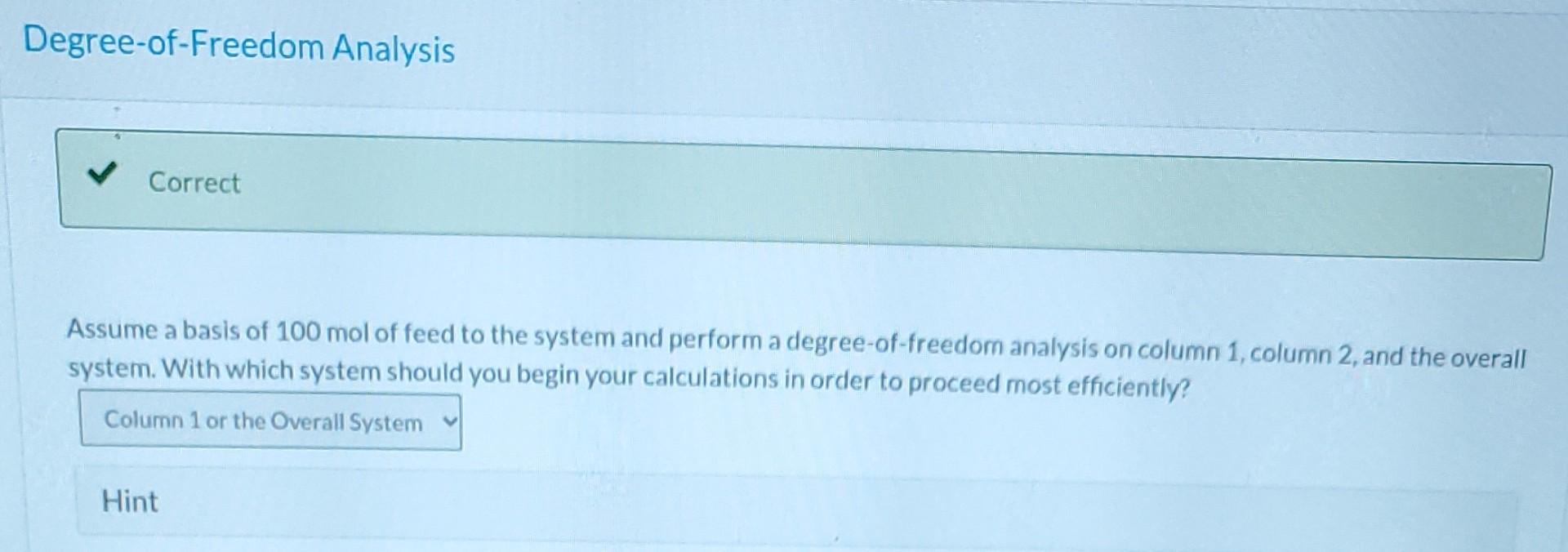

Please answer as much as possible starting from the top. Please.
A liquid stream containing three hydrocarbons is fed to a separation train that separates the mixture into relatively pure components. The feed consists of 39.0 % benzene, 40.0 % toluene, and the remainder xylene, all on a molar basis. The feed enters a distillation column, and two streams exit. The bottoms stream contains 97.0 mole % xylene and no benzene. 96.0% of the xylene in the feed is recovered in the bottoms stream of the first column. The overhead product from the first column is fed to a second distillation column. The distillate (overhead product) from the second column contains 94.0 mole percent benzene and 98.0% of the benzene fed to the column is recovered in this stream. The balance of the overhead stream is toluene. Flow-Chart and Degree-of-Freedom Analysis Draw and label a flow chart for the system. Inspect the flow chart and determine the number of components in each system. Column 1 Column 2 Overall System E i By inspection of the flow chart and implementation of the constraint that the component mole fractions of each stream must add up to 1, determine each of the following mole fractions. Component Mole Fractions Component\Stream Feed Distillate from Column 1 Bottoms from Column 1 Distillate from Column 2 Bottoms from Column 2 Benzene i i Toluene i i 2 Xylene BE i ma Degree-of-Freedom Analysis Correct Assume a basis of 100 mol of feed to the system and perform a degree-of-freedom analysis on column 1 column 2, and the overall system. With which system should you begin your calculations in order to proceed most efficiently? Column 1 or the Overall System Hint Assume a basis of 100 mol of feed and determine the amounts and component mole fractions for the distillate and bottoms of each column. Stream Amounts and Component Mole Fractions Feed Component Stream Distillate from Column 1 n- Bottoms from Column 1 Distillate from Column 2 Bottoms from Column 2 100 n = mol n= i mol mol n = i mol na i mol Benzene 0.390 X = i 0.0 0.940 X= i Toluene 0.400 X= 0.030 0.06 X = Xylene 0.210 X= 0.970 0.0 X=Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


