Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer asap According to the quantum-mechanical model for the hydrogen atom, which of the following electron transitions would produce light with the longer wavelength:
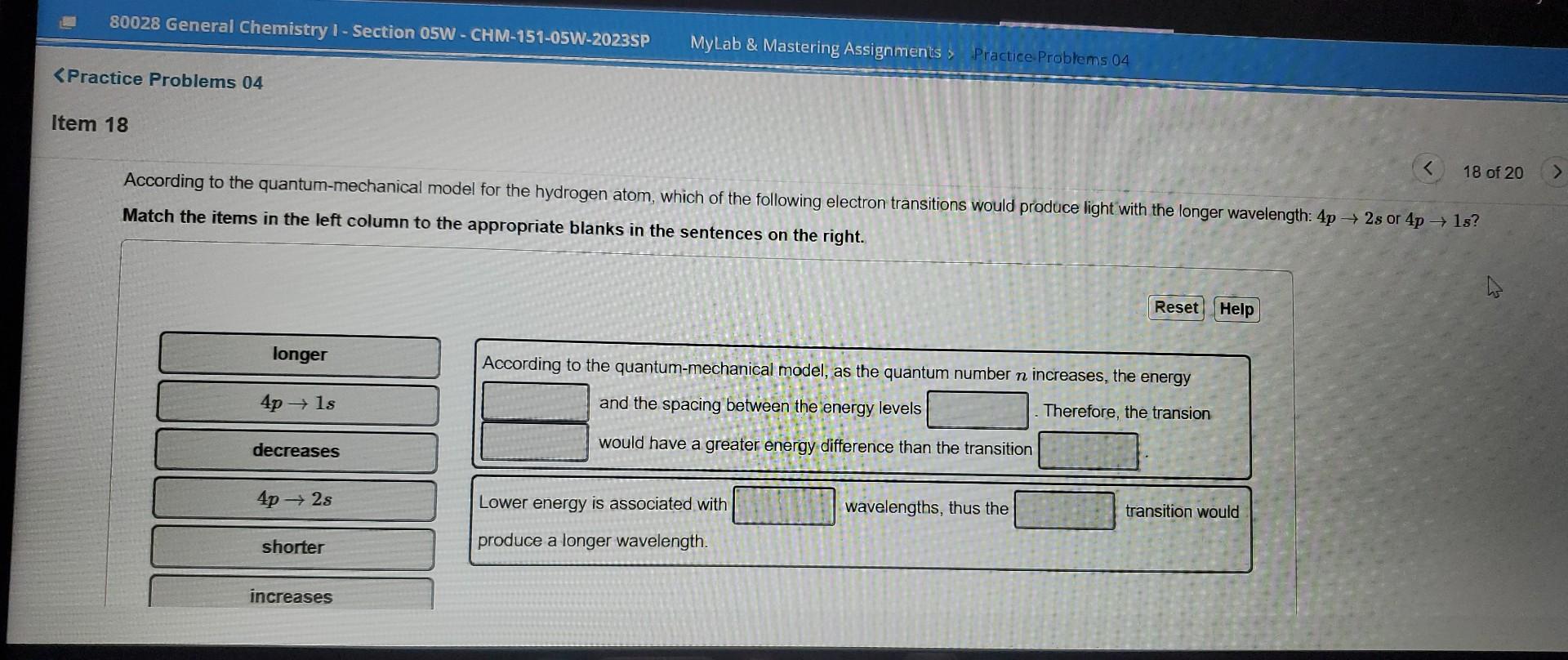
please answer asap
According to the quantum-mechanical model for the hydrogen atom, which of the following electron transitions would produce light with the longer wavelength: 4p2s or 4p1s? Match the items in the left column to the appropriate blanks in the sentences on the right. According to the quantum-mechanical model, as the quantum number n increases, the energy and the spacing between the energy levels Therefore, the transion would have a greater energy difference than the transition Lower energy is associated with wavelengths, thus the produce a longer wavelengthStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


