Answered step by step
Verified Expert Solution
Question
1 Approved Answer
PLEASE ANSWER ASAP When formic acid is heated, it deconposes to bydrogen and carbon dioxide in a first-order decay: HCOOH(g)COO2(g)+Hj(g) The rate of rection is
PLEASE ANSWER ASAP 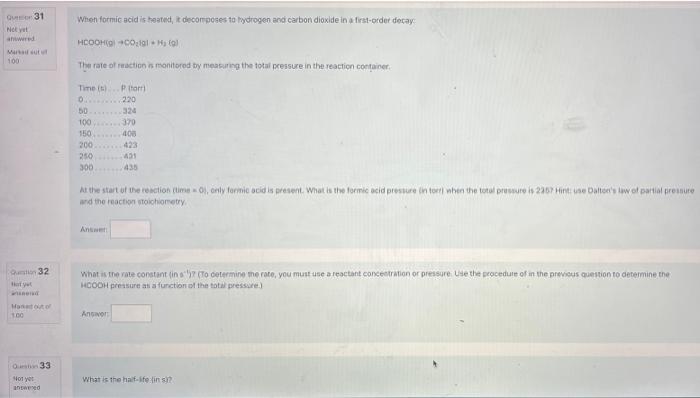
When formic acid is heated, it deconposes to bydrogen and carbon dioxide in a first-order decay: HCOOH(g)COO2(g)+Hj(g) The rate of rection is monitored ty measaing the total pressure in the reaction container. wid the reaction stoichionetry. Antwer! What is the rate constant fin a15 ? (To celermine the rate, you must use a reactant concectration or pressure. Use the procedule of in the artwisus question to deteimine the HoOCH pressure at a function of the totis presssre.) Angavin What is the haif-ifelin al 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


