Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer everything correctly this ia the full question 2. Rate Determining Step of Heterogenous Reactions A certain spherical metal oxide was oxidized by oxygen
please answer everything correctly

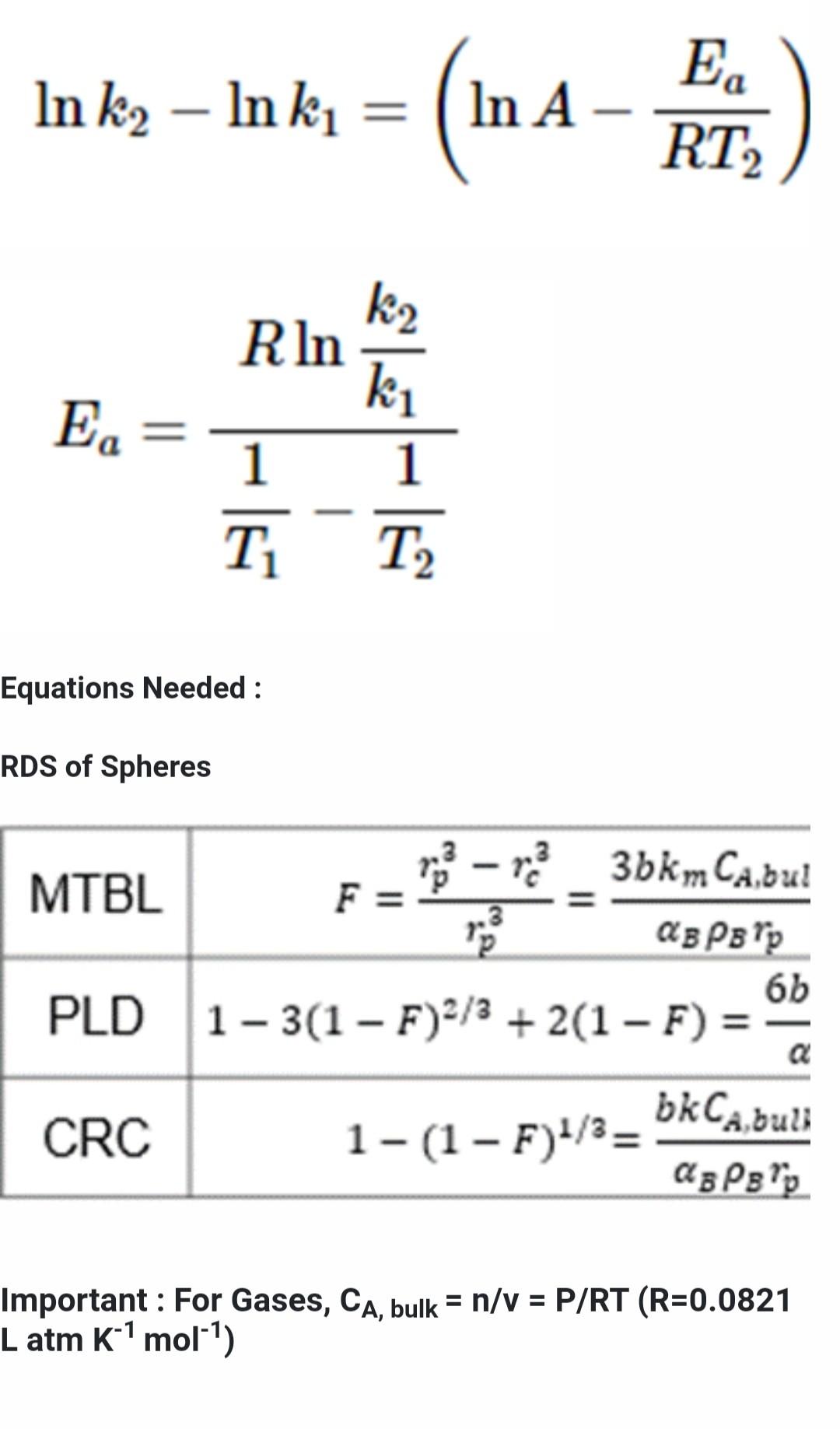
this ia the full question
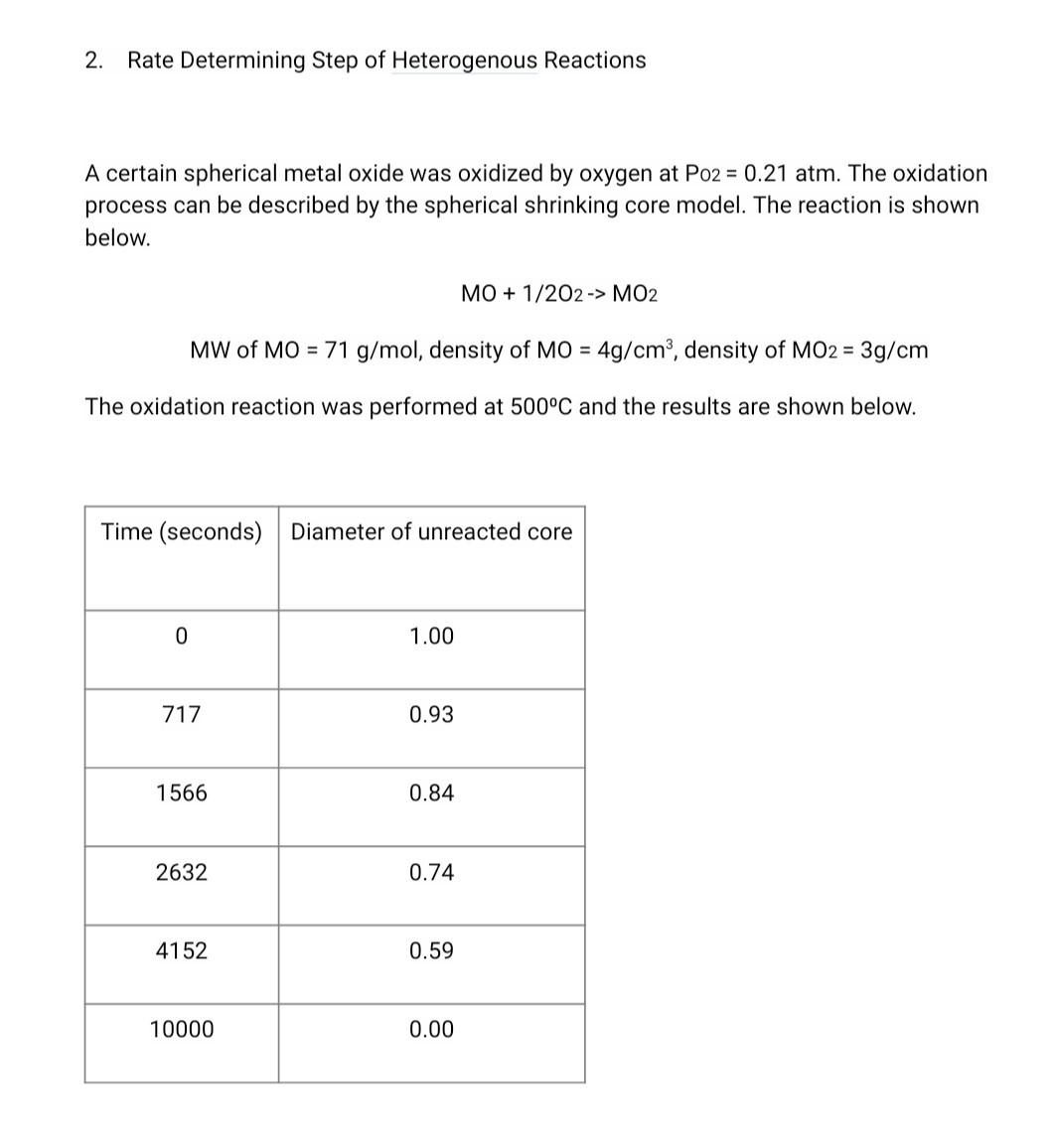
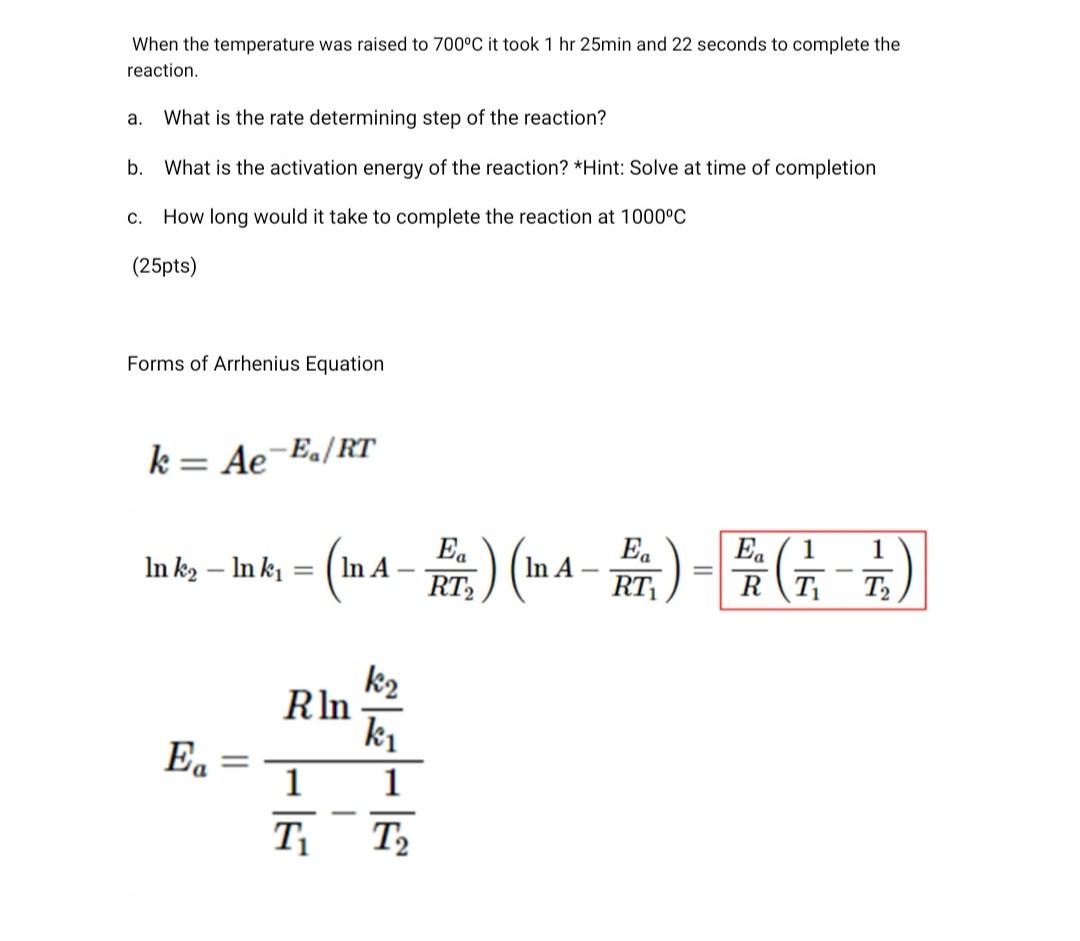
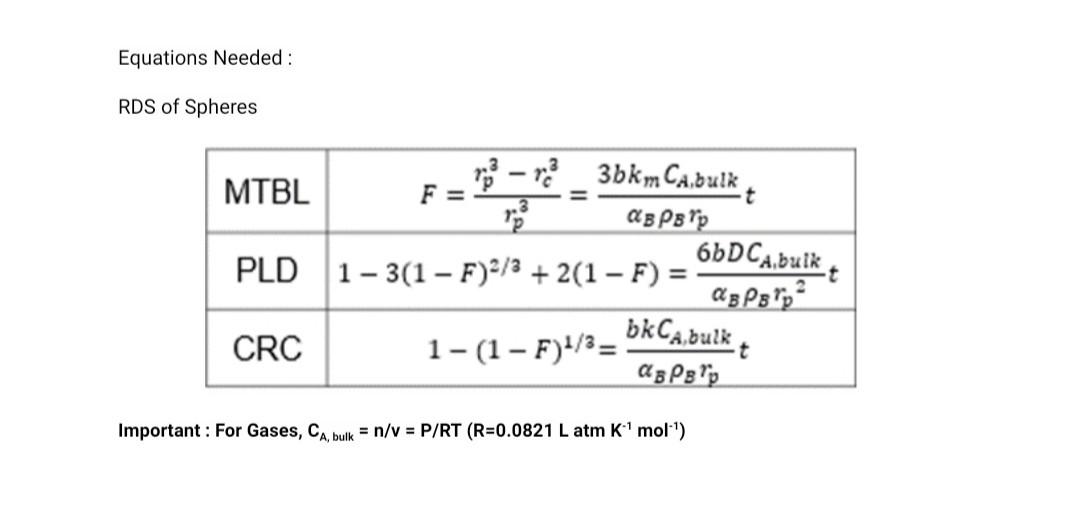
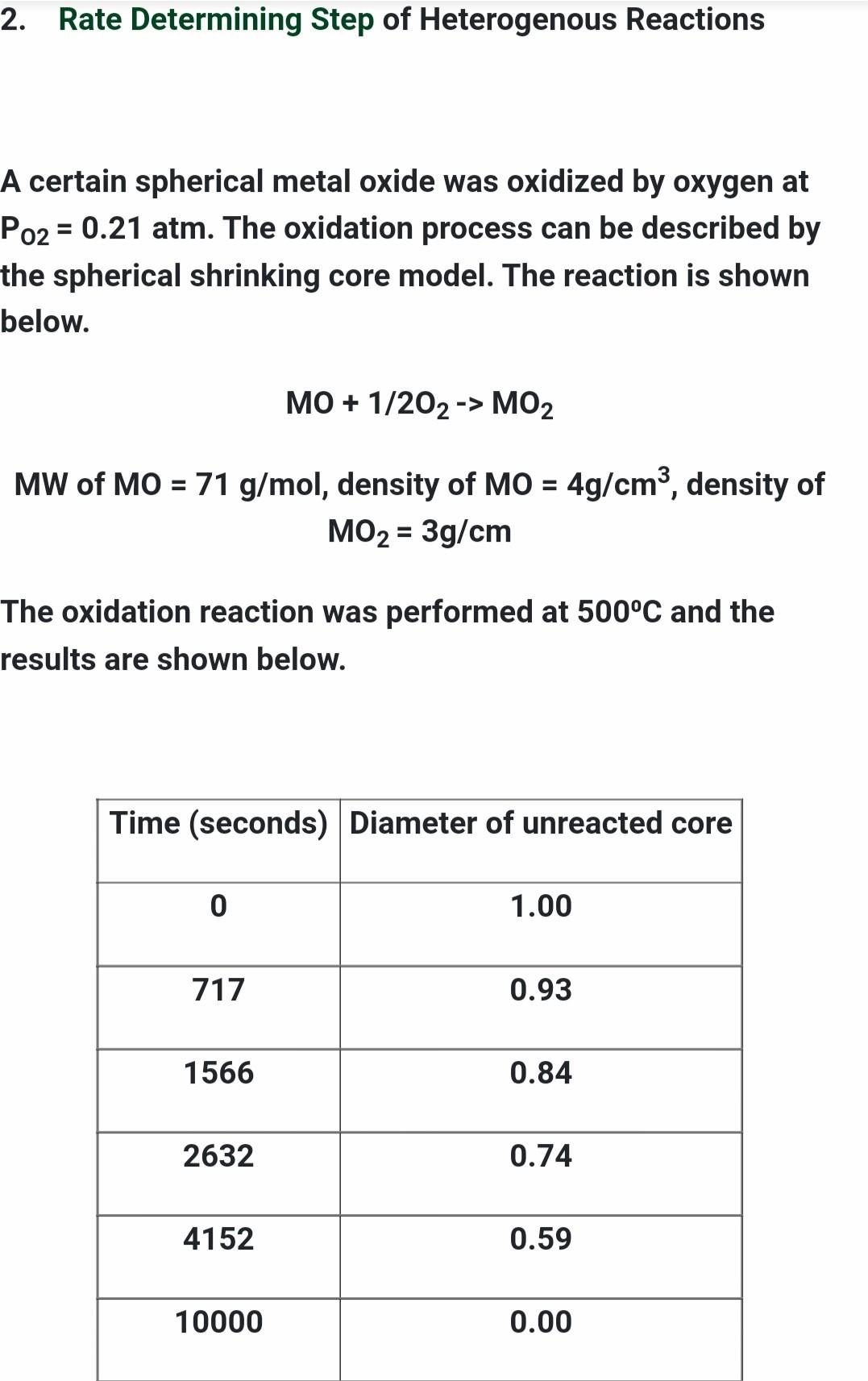
2. Rate Determining Step of Heterogenous Reactions A certain spherical metal oxide was oxidized by oxygen at Po2 = 0.21 atm. The oxidation process can be described by the spherical shrinking core model. The reaction is shown below. MO + 1/2O2 -> MO2 MW of MO = 71 g/mol, density of MO = 4g/cm, density of MO2 = 3g/cm The oxidation reaction was performed at 500C and the results are shown below. Time (seconds) Diameter of unreacted core 0 1.00 717 0.93 1566 0.84 2632 0.74 4152 0.59 10000 0.00 When the temperature was raised to 700C it took 1 hr 25min and 22 seconds to complete the reaction. a. What is the rate determining step of the reaction? b. What is the activation energy of the reaction? *Hint: Solve at time of completion C. How long would it take to complete the reaction at 1000C (25pts) Forms of Arrhenius Equation k= Ae-E/RT E 1 In ku In k, = = (n 4-Fr.) (In A-FE) = 6 7) E 1 RT RT2 RT T2 E. k2 RIn ki 1 1 T2 T Equations Needed: RDS of Spheres t .3 Po - ma_3bkm Cabulk MTBL F= AB PB Pp 66DCA,buik PLD 1-3(1 F)2/3 + 2(1 F) = Ag P8 lp CRC asP'p - 1-(1 F)1/3= bkCa, bulk Important: For Gases, CA, bulk = n/V = P/RT (R=0.0821 L atm k' mol") 2. Rate Determining Step of Heterogenous Reactions = A certain spherical metal oxide was oxidized by oxygen at Po2 = 0.21 atm. The oxidation process can be described by the spherical shrinking core model. The reaction is shown below. MO + 1/202 -> MO2 = MW of MO = 71 g/mol, density of MO = 4g/cm3, density of MO2 = 3g/cm The oxidation reaction was performed at 500C and the results are shown below. Time (seconds) Diameter of unreacted core 0 1.00 717 0.93 1566 0.84 2632 0.74 4152 0.59 10000 0.00 When the temperature was raised to 700C it took 1 hr 25min and 22 seconds to complete the reaction. a. What is the rate determining step of the reaction? b. What is the activation energy of the reaction? *Hint: Solve at time of completion c. How long would it take to complete the reaction at 1000C (25pts) Forms of Arrhenius Equation k= Ae-Ea/RT In k2 Inki = = (n- In A E RT k2 R In ki E In k2 - In ki = (WA- In A E. RT E. k2 R In ki 1 1 T T Equations Needed : RDS of Spheres F = .3 - 3 3bkm Cabul MTBL @BP 6b PLD 1-3(1 F)2/3 + 2(1 F) = bk Cabul CRC 1-(1 F)1/3= ? Important: For Gases, CA, bulk = n/v = P/RT (R=0.0821 L atm K-1 mol-1)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


