PLEASE ANSWER FAST, This is a PCHEM problem below,please show all work clearly and use correct units in answer
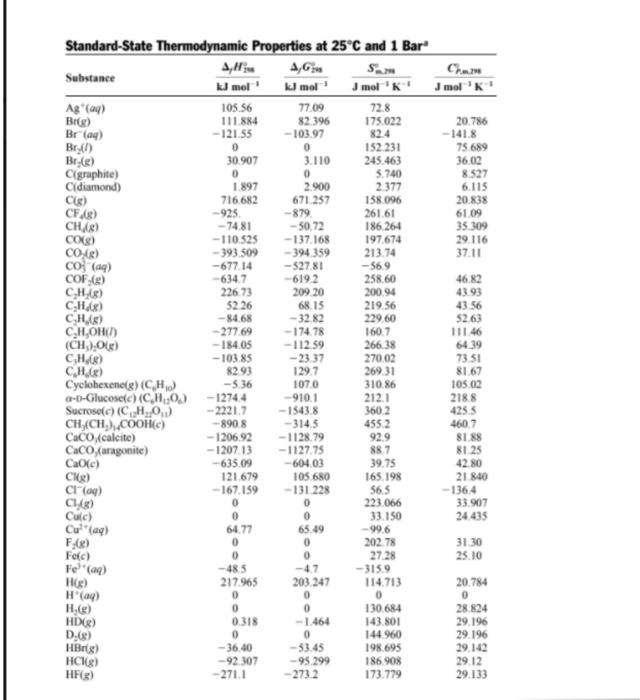
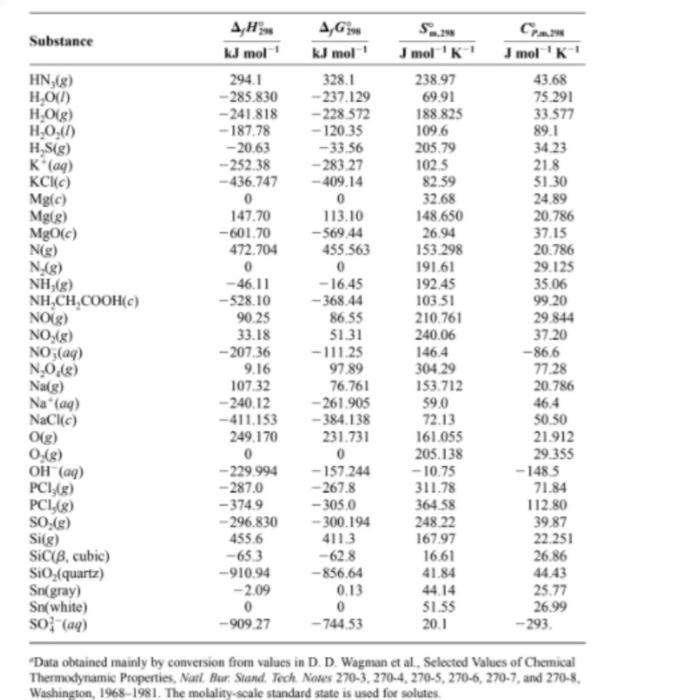
Use data from the thermodynamic tables and neglect temperature dependence of Acp to find AH 700 for: 2HN3 (9) + 2NO (9) H202 (1) + 4N2 (9) J mol' 0 CH/B) Standard-State Thermodynamic Properties at 25C and 1 Bar A,G Substance kJ mol' kJ mol mol 'K! Ag (ay) 105.56 77.09 72.8 Bilge) 111.884 82.396 175022 Bray) -121.55 -103.97 82.4 Br.) 0 0 152 231 Br(e) 30907 3.110 245.463 Cigraphite) 0 5.740 diamond) 1.897 2.900 2.377 Cig) 716682 671.257 158.096 CF.8) -925 -879 261.61 -74.81 -50.72 186.264 CO(g) -110.525 -137.168 197.674 COC) -393.509 -394 359 213.74 co (aq) --677.14 -527.81 -569 COF.(e) -619.2 258.60 CHAS) 226.73 209 20 200.94 CH.8) 52.26 68.15 219.56 C.HS) -84.68 - 3282 229.60 CH,OHC) -277.69 - 17478 160.7 (CH) Og) -184.05 -112.59 266.38 CHAS) -103.85 -2337 270.02 CH.) 82.93 129.7 269.31 Cyclohexene(g) (CH) -5.36 1070 310.86 a-D-Glucosee) (CH20.) - 1274.4 -910.1 212.1 Sucrosec) (CH_0.) -22217 - 1543.8 360.2 CH_(CH),COOH) -8908 -3145 4552 CaCO(calcite) -1206.92 -1128.79 92.9 Caco (aragonite) -120713 -1127.75 88.7 Ca(c) - 635.09 -604.03 39.75 CHO) 121.679 105.680 165.198 Cl(aq) - 167.159 - 131 228 56.5 C1 (8) 0 0 223.066 Cuc) 0 0 33.150 Cwa 64.77 65.49 -99.6 0 0 202.78 Fec) 0 27.28 Fe (aq) -485 -47 -3159 He) 217.965 203.247 114713 H(aq) 0 0 0 H.) 0 0 130.684 HD(R) 0318 -1.464 143801 D.(8) 0 144 960 HBrg) -36.40 -53.45 198.695 HCR) -92 307 -95.299 186.908 HF) -271.1 273.2 173.779 20.786 - 141.8 75.689 36.00 8.527 6.115 20.838 61 09 35309 29.116 37.11 46.82 4393 43.56 5263 111.46 64.39 73.51 81.67 10502 2188 4255 460.7 81.88 8125 42.80 21840 -136.4 33 907 24.435 31.30 25.10 20.784 0 28 824 29.196 29.196 29.142 29.12 29.133 FAX) 0 Substance HN (g) HO(1) H.O(g) H.O.(1) H.S(g) K. (aq) KCC) Mg(c) Mg(s) MgO(c) N(g) N.(8) NH,g) NH,CH,COOH(c) NOLE) NO (8) NO3(aq) NO,R) AH KJ mol 294.1 -285.830 -241.818 -187.78 -20.63 - 252.38 - 436.747 0 147.70 -601.70 472.704 0 -46.11 - 528.10 90.25 33.18 -207.36 9.16 107.32 - 240.12 - 411.153 249.170 0 -229.994 -287.0 -3749 -296.830 455.6 -65.3 -910.94 -2.09 0 -90927 4,6 KJ mol 328.1 -237.129 -228.572 - 120.35 - 33.56 -283.27 -409.14 0 113.10 --569.44 455,563 0 -16.45 -368.44 86.55 51.31 - 111.25 97.89 76.761 - 261.905 -384.138 231.731 0 -157.244 -267.8 -305.0 -300.194 411.3 -62.8 - 856,64 0.13 0 - 744 53 Jmol 'K 23897 69.91 188,825 109.6 205.79 102.5 82.39 32.68 148,650 26.94 153.298 191.61 192.45 103.51 210.761 240.06 146.4 304.29 153.712 J mol 'K! 43.68 75.291 33.577 89.1 34.23 21.8 51.30 24.89 20.786 37.15 20.786 29.125 35.06 99.20 29.844 37.20 -86.6 77.28 20.786 46.4 50.50 21.912 29.355 -148,5 71.84 112.80 39.87 22.251 26.86 44.43 25.77 26.99 -293. Nag) 59,0 Na'(a) NaCl) O(g) 0.18) OH(aq) PC148) PCI,(8) so) Si[g) SiCiB, cubic) Sio, (quartz) Sn(gray) Sn(white) SO" (aq) 72.13 161.055 205.138 -10.75 311.78 364.58 248.22 16797 16.61 41.84 51.55 20.1 Data obtained mainly by conversion from values in D.D. Wagman et al., Selected Values of Chemical Thermodynamic Properties, Natl. Bur Stand. Tech. Nores 270-3.270-4, 270-5, 270-6, 270-7, and 270-8 Washington, 1968-1981. The molality-scale standard state is used for solutes