Question: Please answer in the picture Problem 11.39 not Problem 11.38. I just needed to confirm something as to how to approach and answer the problem.
Please answer in the picture Problem 11.39 not Problem 11.38. I just needed to confirm something as to how to approach and answer the problem. This would help me in my understanding and studying, thank you:)
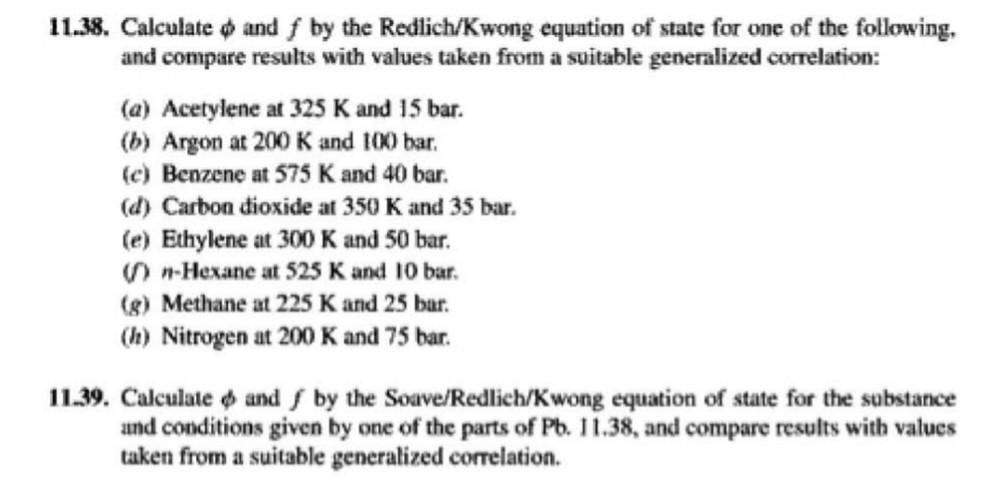
11.38. Calculate and f by the Redlich/Kwong equation of state for one of the following, and compare results with values taken from a suitable generalized correlation: (a) Acetylene at 325 K and 15 bar. (b) Argon at 200 K and 100 bar. (c) Benzene at 575 K and 40 bar. (d) Carbon dioxide at 350 K and 35 bar. (e) Ethylene at 300 K and 50 bar. -Hexane at 525 K and 10 bar. (8) Methane at 225 K and 25 bar. (1) Nitrogen at 200 K and 75 bar. 11.39. Calculate o and by the Soave/Redlich/Kwong equation of state for the substance and conditions given by one of the parts of Pb. 11.38, and compare results with values taken from a suitable generalized correlation. 11.38. Calculate and f by the Redlich/Kwong equation of state for one of the following, and compare results with values taken from a suitable generalized correlation: (a) Acetylene at 325 K and 15 bar. (b) Argon at 200 K and 100 bar. (c) Benzene at 575 K and 40 bar. (d) Carbon dioxide at 350 K and 35 bar. (e) Ethylene at 300 K and 50 bar. -Hexane at 525 K and 10 bar. (8) Methane at 225 K and 25 bar. (1) Nitrogen at 200 K and 75 bar. 11.39. Calculate o and by the Soave/Redlich/Kwong equation of state for the substance and conditions given by one of the parts of Pb. 11.38, and compare results with values taken from a suitable generalized correlation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


