Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answer it immediately table 2 please answer all. II. Calculate AH, for each of the following (using Table 1). 1. C H20) from the

please answer it immediately
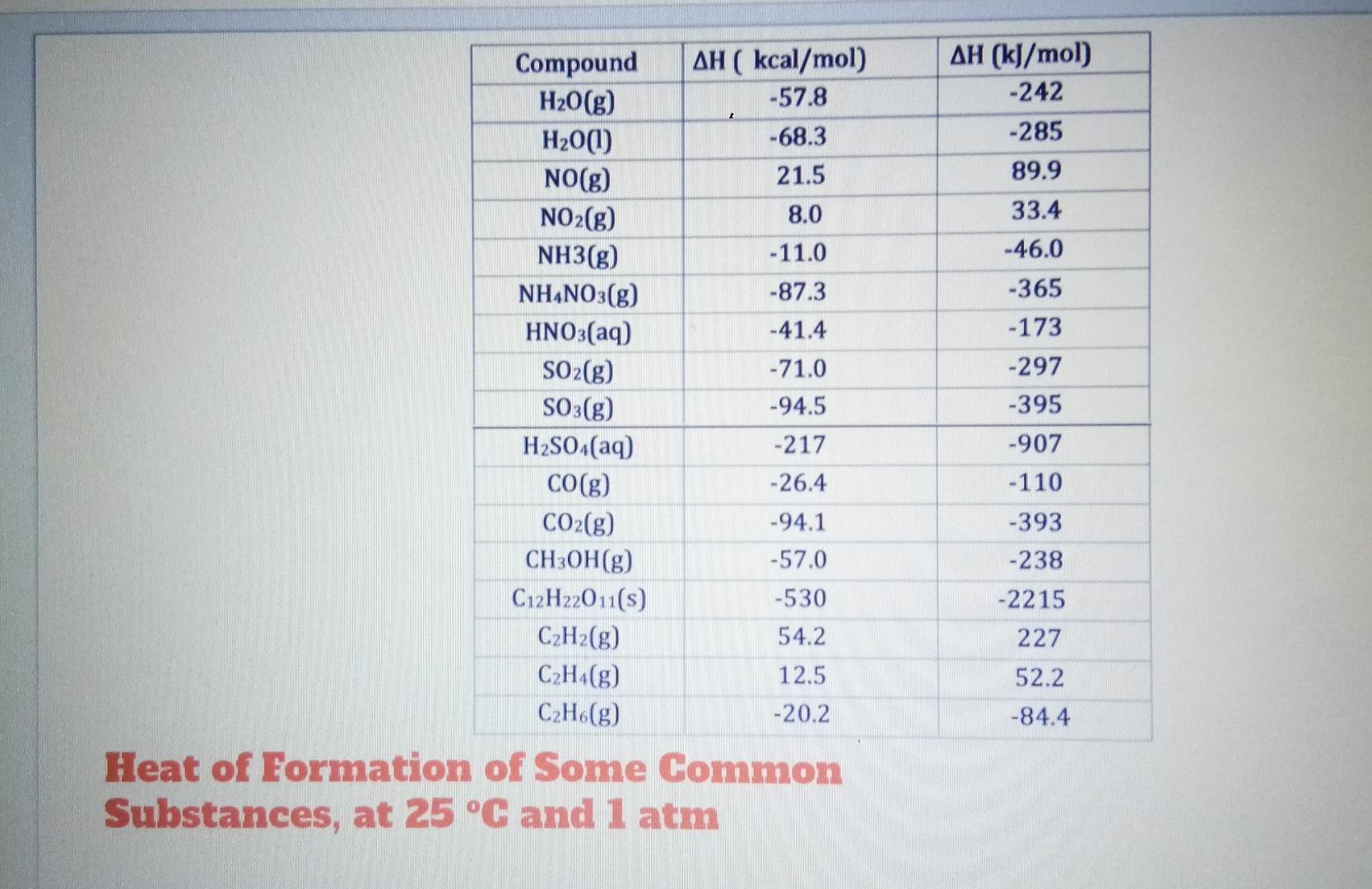
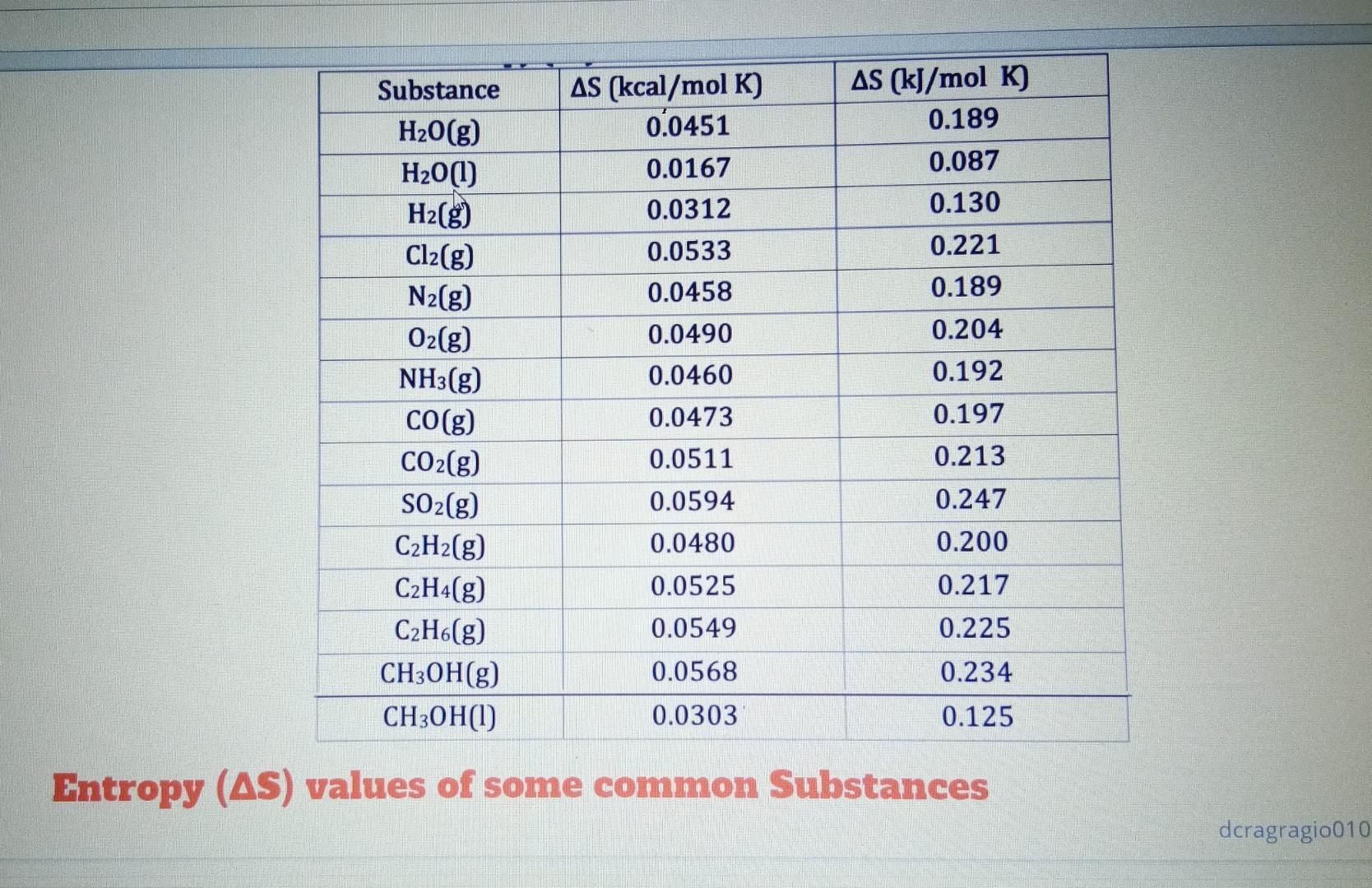
table 2
please answer all.
II. Calculate AH, for each of the following (using Table 1). 1. C H20) from the reaction 2 C2H2(g) + 502(g) 4 CO2(g) + 2 H2O(g) + 600kcal CH (w from the reaction CH.(g) + 2 02(8) - CO2(g) + 2 H20(g) + 192 kcal III. Given the following reactions k. S + 0,+ SO2 + 71.0 kcal 2 502 + 02 2 SO3 + 47.0 kcal Calculate AH for 2 S + 302 2 SO 12 C + O2 + 2 CO + 52.8 kcal 2 CO + 0 2 CO: + 125 kcal Calculate AH, for C + 02 -+ CO2 I IV. Calculate AH, AS and AG at 25 C, and tell if the reaction will go spontaneously (use Table 1 and 2) m. N.(g) + 3 H. (8) 2 NH (3) n. 2 CO(g) + O2(g) - 2 CO2(g) o 2 CH2(g) + 50 (8) 4 CO.(8) + 2 H 00g) AH (kJ/mol) -242 -285 89.9 33.4 -46.0 -365 Compound AH ( kcal/mol) H20(g) -57.8 H20(1) -68.3 NO(g) 21.5 NO2(g) 8.0 NH3(g) - 11.0 NH4NO3(g) -87.3 HNO3(aq) -41.4 SO2(g) - 71.0 SO3(g) -94.5 H2SO4(aq) -217 CO(g) -26.4 CO2(g) -94.1 CH3OH(g) -57.0 C12H22011(s) -530 C2H2(g) 54.2 C2H4(g) 12.5 C2H.(g) -20.2 Heat of Formation of Some Common Substances, at 25 C and 1 atm -173 -297 -395 -907 -110 -393 -238 -2215 227 52.2 -84.4 AS (kJ/mol K) 0.189 0.087 0.130 0.221 0.189 Substance H2O(g) H20(1) H2(g) Cl2(g) N2(g) O2(g) NH3(g) CO(g) CO2(g) SO2(g) C2H2(g) C2H4(g) C2H6(g) CH3OH(g) CH3OH(1) AS (kcal/mol K) 0.0451 0.0167 0.0312 0.0533 0.0458 0.0490 0.0460 0.0473 0.0511 0.0594 0.0480 0.0525 0.204 0.192 0.197 0.213 0.247 0.200 0.217 0.0549 0.225 0.0568 0.234 0.0303 0.125 Entropy (AS) values of some common Substances dcragragio010Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


