Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer recovery. All answers before are correct. Please use them. Answer for percent of B is correct, find percent of T. A liquid mixture
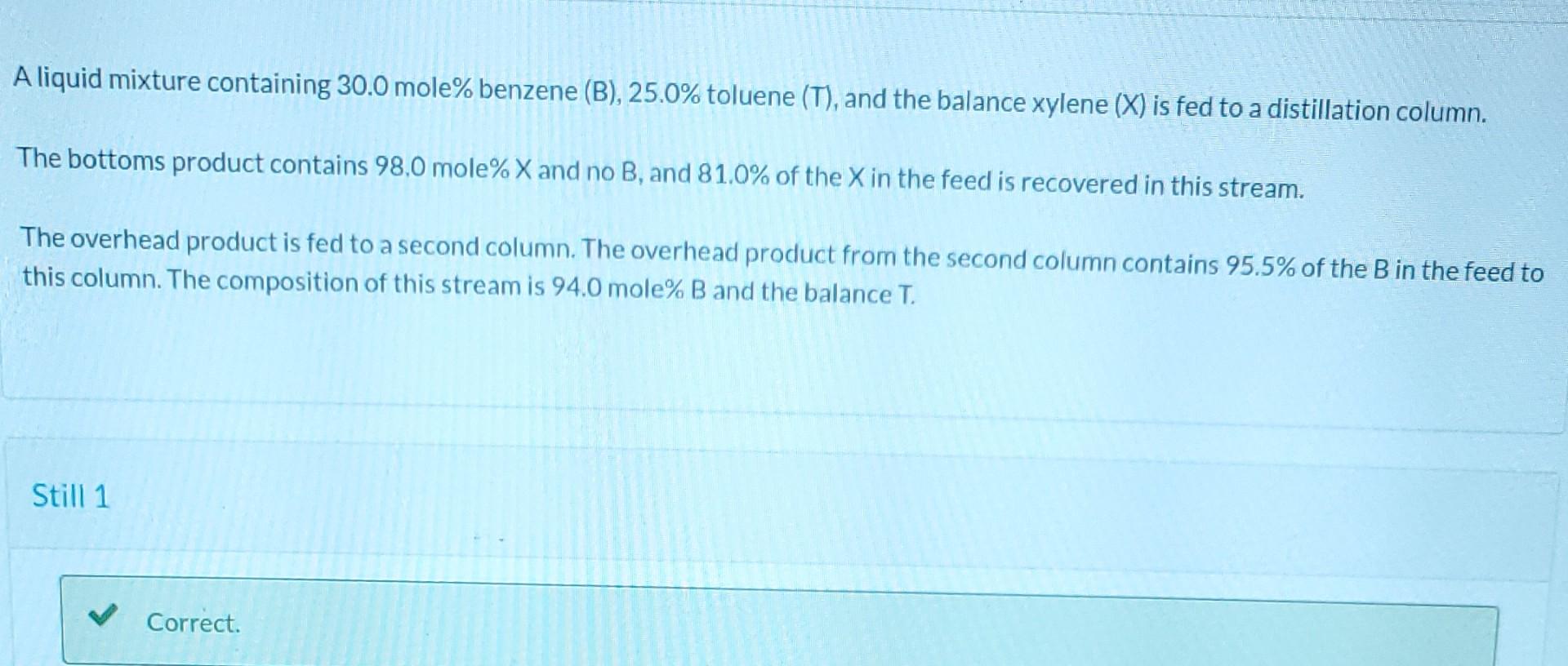


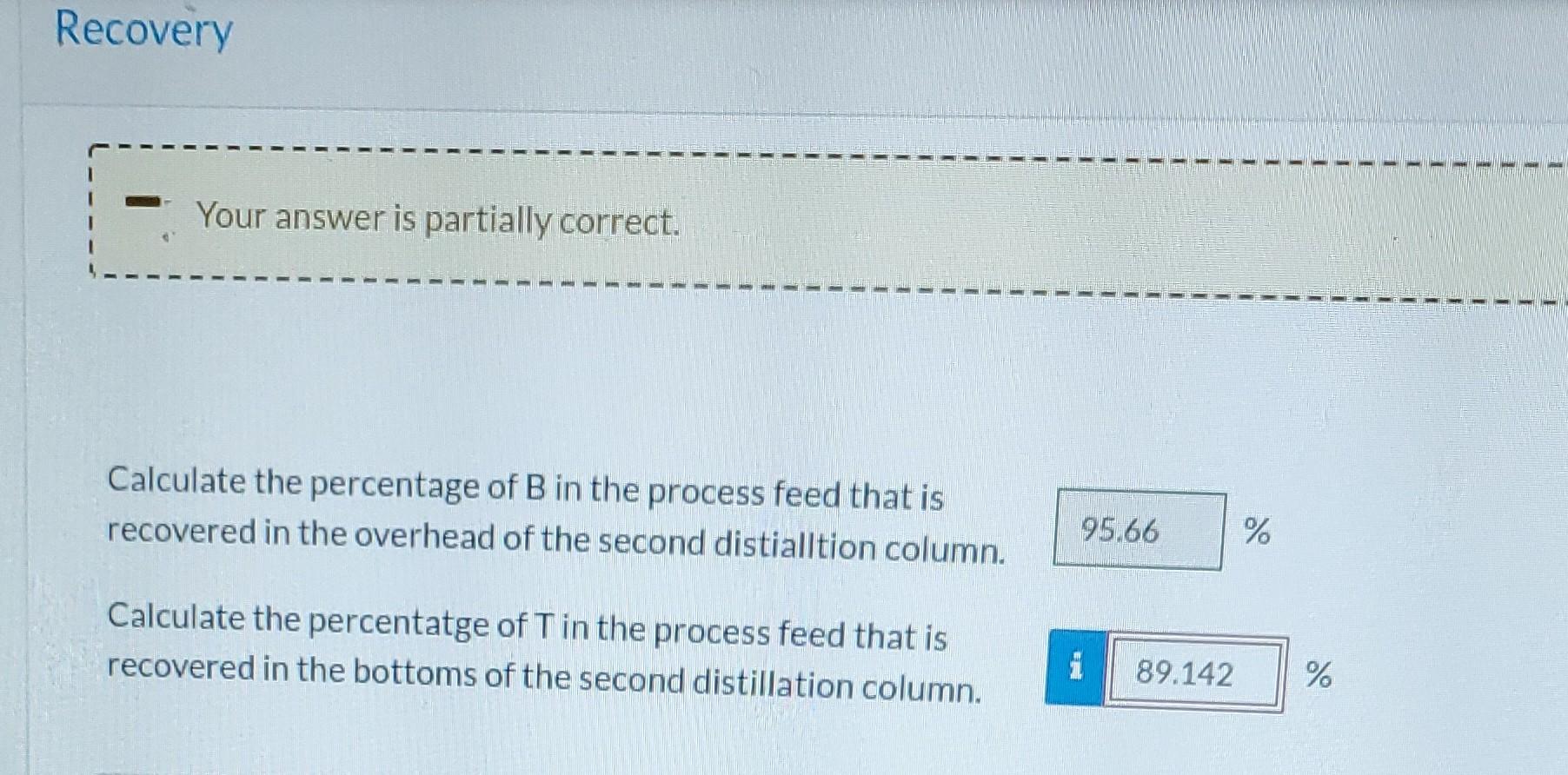
Please answer recovery. All answers before are correct. Please use them. Answer for percent of B is correct, find percent of T.
A liquid mixture containing 30.0 mole% benzene (B), 25.0% toluene (T), and the balance xylene (X) is fed to a distillation column. a The bottoms product contains 98,0 mole% X and no B, and 81.0% of the X in the feed is recovered in this stream. The overhead product is fed to a second column. The overhead product from the second column contains 95.5% of the B in the feed to this column. The composition of this stream is 94.0 mole% B and the balance T. Still 1 Correct. Write three balances and use one constraint for the performance of Still 1 to solve for the following quantities for a process feed ni) of 200.0 mol/h. First Still Bottoms flow rate (12) = 74.388 mol/h Overhead fow rate (13) = 125.61 mol/h Benzene mole fraction in overhead (X38) = 0.478 Toluene mole fraction in overhead (x3r) = 0.386 Touth Write three balances and use one constraint for the performance of Still 2 to solve for the following quantities for a proce: 11) of 200.0 mol/h. Second Still Overhead from second still (15) = 61 mol/h Bottoms from second still (14) = 64.61 mol/h Benzene mole fraction in bottoms from second still (4B) = 0.0418 Toluene mole fraction in bottoms from second still (4T) = 0.694 Recovery 1 11 1 . 1 1 1 1 1 1 1 1 1 Your answer is partially correct. - Calculate the percentage of B in the process feed that is recovered in the overhead of the second distialltion column. 95.66 % Calculate the percentatge of Tin the process feed that is recovered in the bottoms of the second distillation column. i 89.142 %Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


