Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please answer the reaction engineering problem accurately. Do not copy other's work and do not attempt if unsure! The following data on baker's yeast in
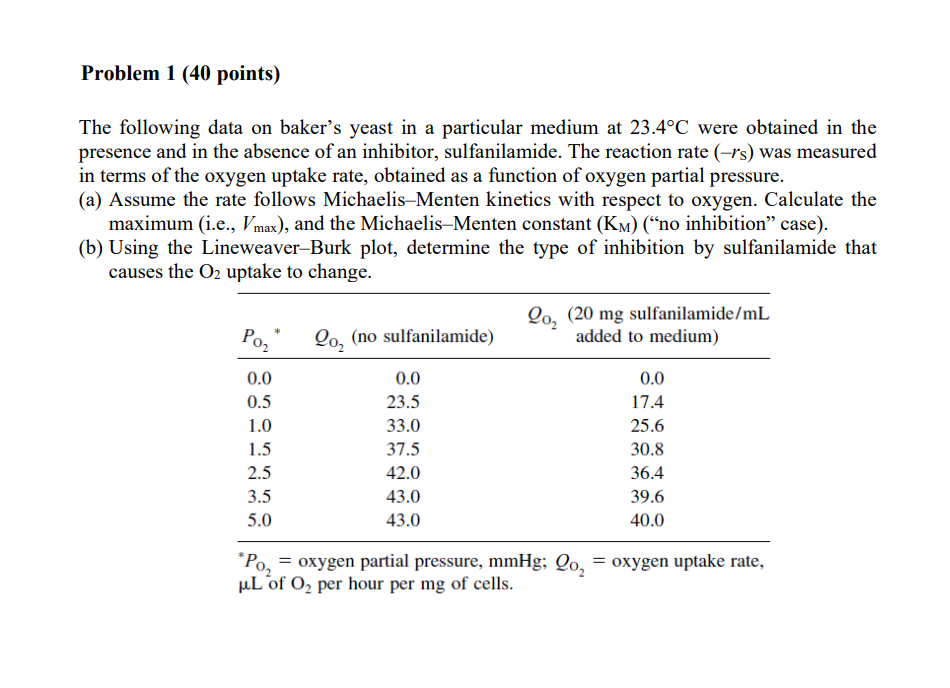
Please answer the reaction engineering problem accurately. Do not copy other's work and do not attempt if unsure!
The following data on baker's yeast in a particular medium at 23.4C were obtained in the presence and in the absence of an inhibitor, sulfanilamide. The reaction rate (rs) was measured in terms of the oxygen uptake rate, obtained as a function of oxygen partial pressure. (a) Assume the rate follows Michaelis-Menten kinetics with respect to oxygen. Calculate the maximum (i.e., Vmax ), and the Michaelis-Menten constant (KM) ("no inhibition" case). (b) Using the Lineweaver-Burk plot, determine the type of inhibition by sulfanilamide that causes the O2 uptake to change. PO2= oxygen partial pressure, mmHg;QO2= oxygen uptake rate, L2 of O2 per hour per mg of cellsStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


