Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please answrr all 4 A sample of Ca weighs 59.9 grams. Will a sample of He that contains the same number of atoms weigh more
please answrr all 4 


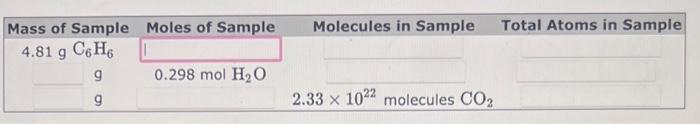
A sample of Ca weighs 59.9 grams. Will a sample of He that contains the same number of atoms weigh more or less than 59.9 grams? A sample of He weighs less than 59.9 grams. A sample of He weighs more than 59.9 grams. Calculate the mass of a sample of He that contains the same number of atoms. Mass =gHe A sample of dichlorodibromosilane, SiCl2Br2, contains 0.0456 mol of the compound. What is the mass of the sample, in grams? 9 A sample of chloroethane, C2H5Cl, has a mass of 16.3g. What amount, in moles, does this mass represent? mol \begin{tabular}{|c|c|c|c|c|c|} \hline Mass of Sample & Moles of Sample & \\ \hline 4.81gC6H6 & & \\ g & 0.298molHH2O & \\ g & & 2.331022 molecules CO2 \\ \hline \end{tabular} 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


