Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please EXPLAIN and solve EACH / ALL part(s) in Question #7 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #7!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEW TO CHEMISTRY! I AM A COMPLETE NEWBIE!
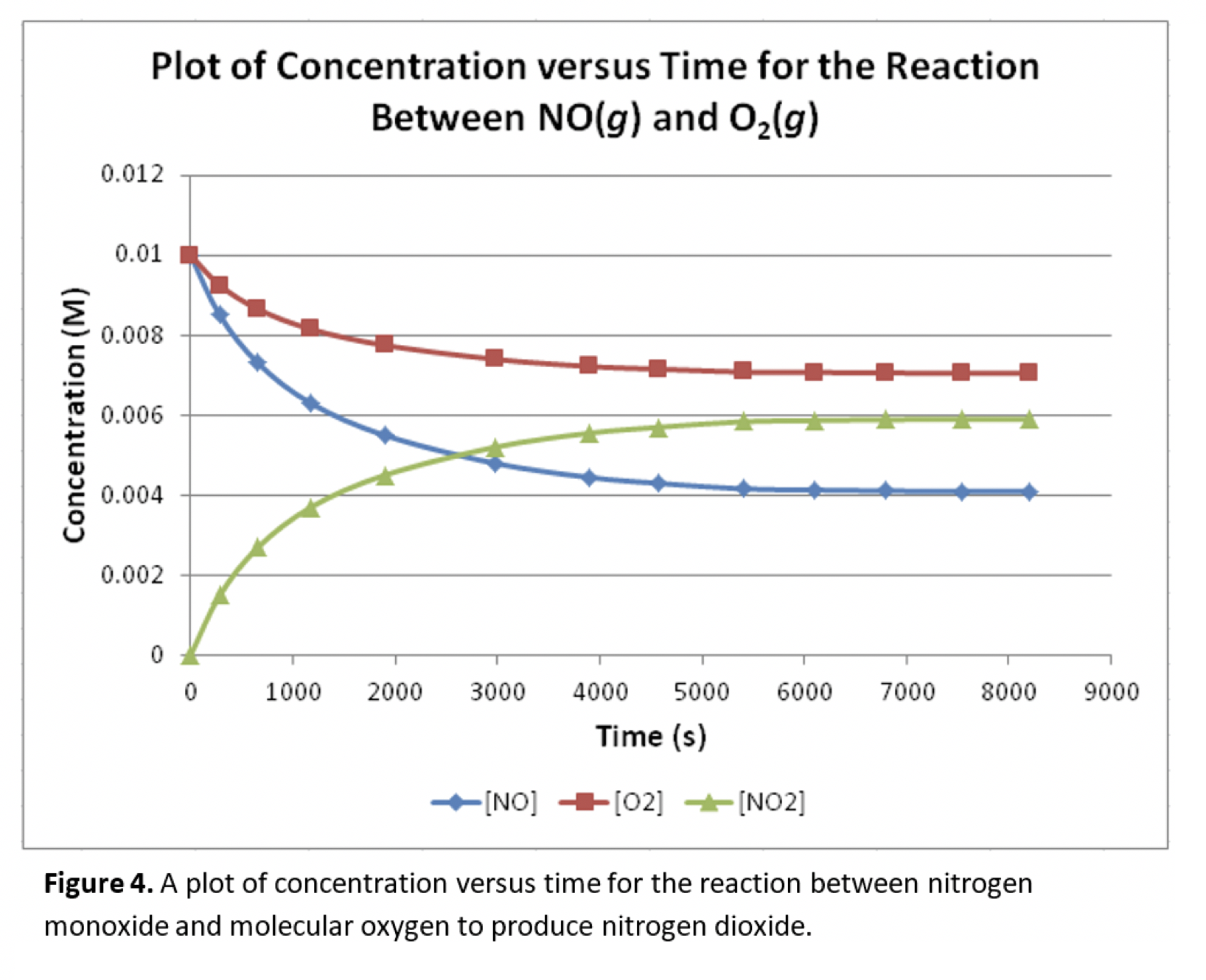
Figure 4. A plot of concentration versus time for the reaction between nitrogen monoxide and molecular oxygen to produce nitrogen dioxide. Part 3 - Factors Affecting Reaction Rates 7A. What are the major factors that affect reaction rates? Type your answer here. In order for a chemical reaction to occur, reactant molecules must collide with each other. Keep this in mind as you answer the following questions. 7B. Looking at Figure 4 again, we see that the rate of reaction slows as the reaction proceeds. How is the change in concentration of reactant (NO2O2) related to the change in the rate of reaction? Type your answer here. 7C. Based on your answer to 7B, why might the rate of reaction decrease and the amount of reactant decreases? How are these two things related to each other? Type your answer here. 7D. According to our textbook, increasing the temperature increases the rate of a chemical reaction. Thinking in terms of molecules colliding again, how might temperature play a role in increasing the reaction rate? Type your answer here
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


