Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Please EXPLAIN and solve EACH / ALL part(s) in Question #7 ! DOUBLE CHECK YOUR WORK AND ANSWER(S) . PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS
Please EXPLAIN and solve EACH/ALL part(s) in Question #7!
DOUBLE CHECK YOUR WORK AND ANSWER(S).
PLEASE NEATLY SHOW ALL WORK, EXPLANATIONS, & CALCULATIONS STEP-BY-STEP USING PEN AND PAPER! I AM NEWTO CHEMISTRY! I AM A COMPLETE NEWBIE!
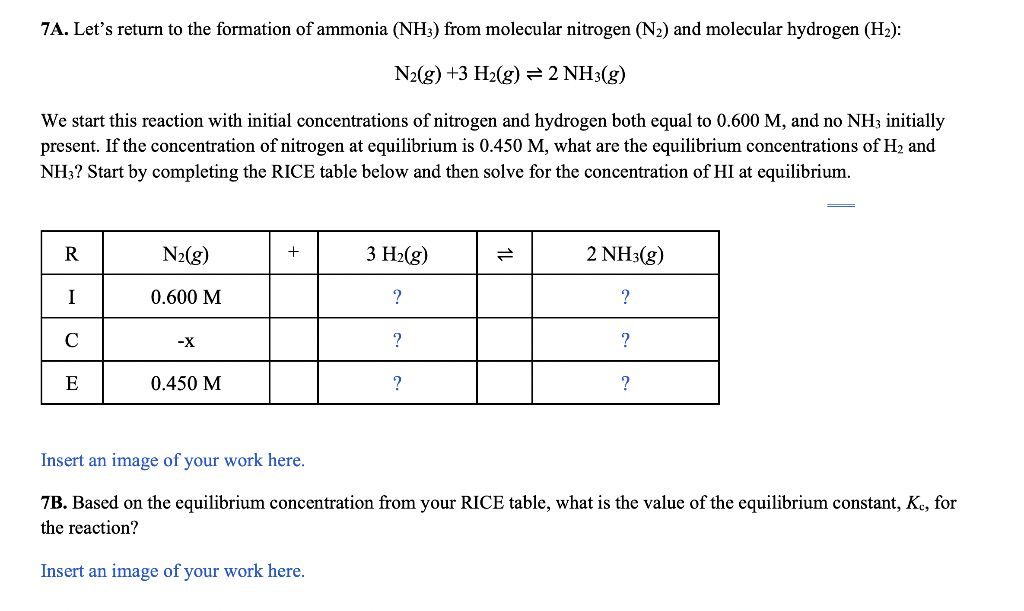
N2(g)+3H2(g)2NH3(g) We start this reaction with initial concentrations of nitrogen and hydrogen both equal to 0.600M, and no NH3 initially present. If the concentration of nitrogen at equilibrium is 0.450M, what are the equilibrium concentrations of H2 and NH3 ? Start by completing the RICE table below and then solve for the concentration of HI at equilibrium. Insert an image of your work here. 7B. Based on the equilibrium concentration from your RICE table, what is the value of the equilibrium constant, Kc, for the reaction? Insert an image of your work here
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


