Answered step by step
Verified Expert Solution
Question
1 Approved Answer
2. The ore with the composition given below, is roasted and sulfur (S) amount is reduced to 6.8%.5% of the Cu becomes CuO, and the
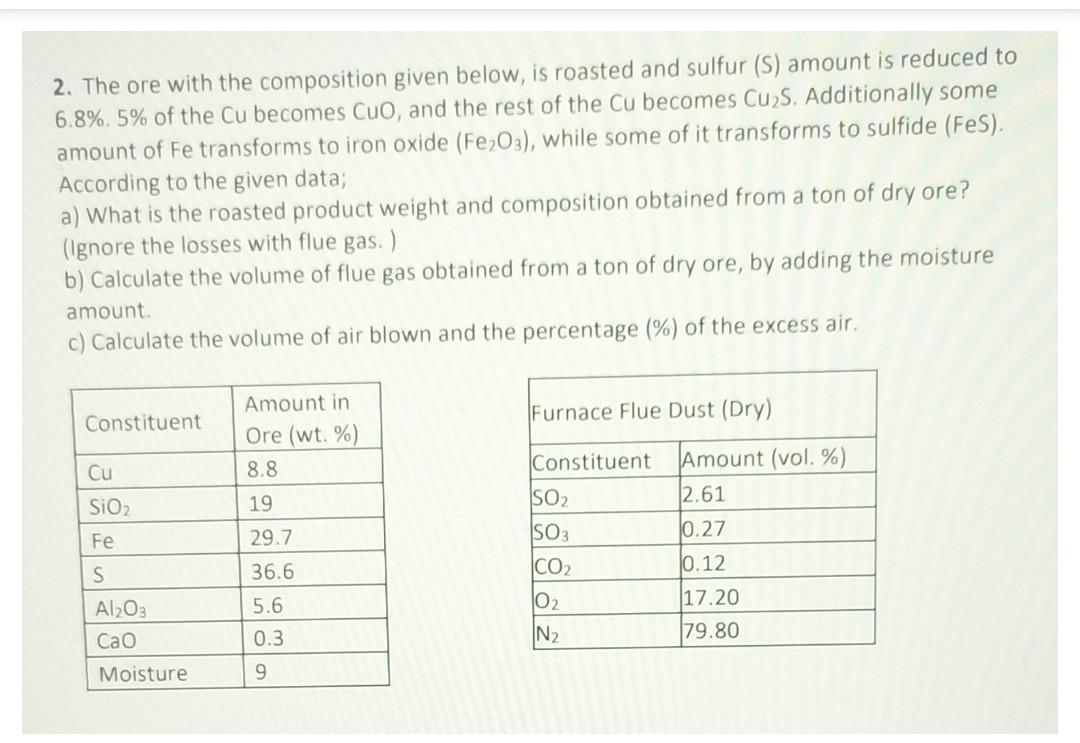
2. The ore with the composition given below, is roasted and sulfur (S) amount is reduced to 6.8%.5% of the Cu becomes CuO, and the rest of the Cu becomes Cu2S. Additionally some amount of Fe transforms to iron oxide (Fe2O3), while some of it transforms to sulfide (FeS). According to the given data; a) What is the roasted product weight and composition obtained from a ton of dry ore? (Ignore the losses with flue gas. ) b) Calculate the volume of flue gas obtained from a ton of dry ore, by adding the moisture amount. c) Calculate the volume of air blown and the percentage (\%) of the excess air
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


