Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please explain what's the effect of the neighboring group on the rate of reaction by using chair form please brief all steps in Details by
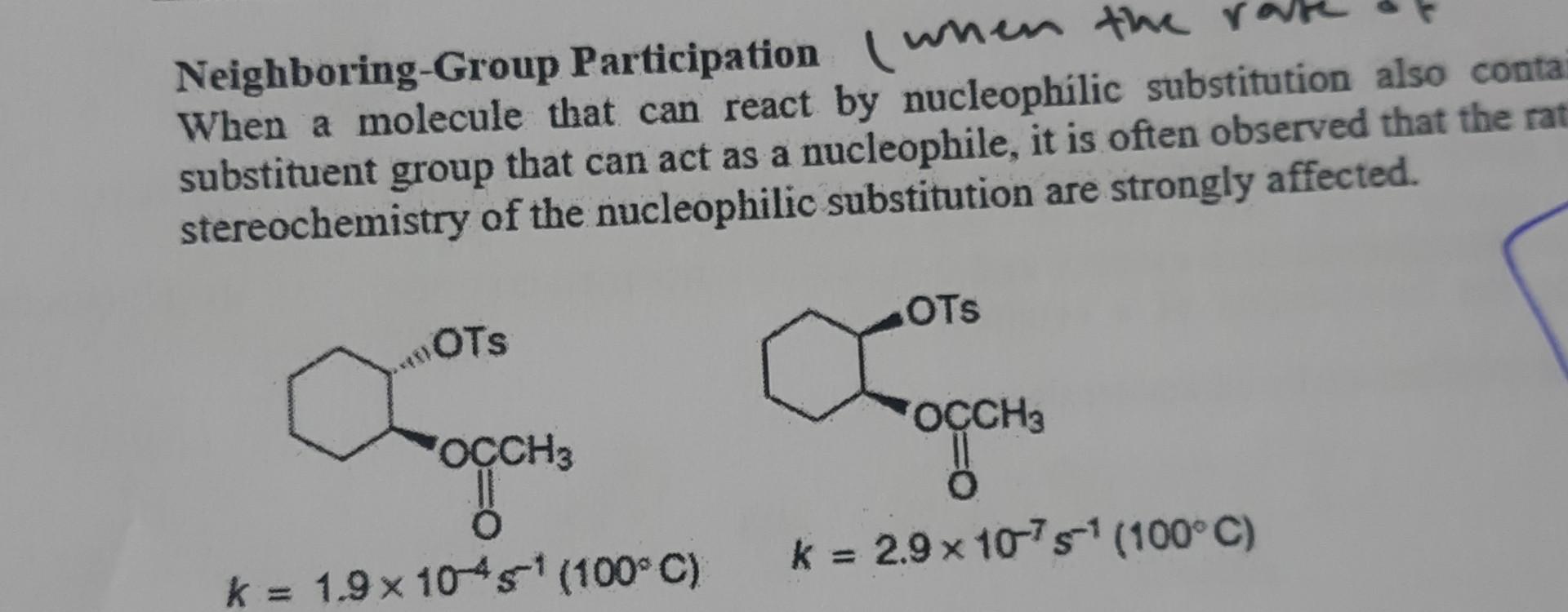
please explain what's the effect of the neighboring group on the rate of reaction by using chair form please brief all steps in Details
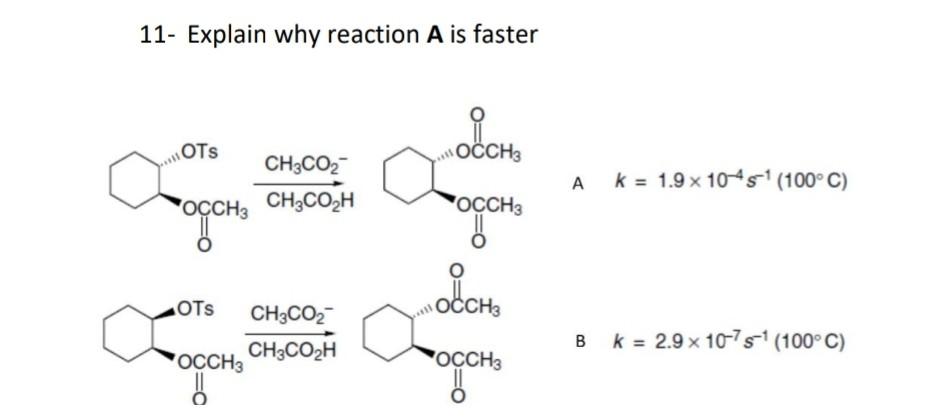
by using cyclohexan chairform explained the answer
Neighboring-Group Participation (when the When a molecule that can react by nucleophilic substitution also conta substituent group that can act as a nucleophile, it is often observed that the rat stereochemistry of the nucleophilic substitution are strongly affected. 11- Explain why reaction A is faster CH3CO2HCH3CO2 A k=1.9104s1(100C) CH3CO2HCH3CO2 B k=2.9107s1(100C)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


