Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please explain, why we said that is saturated in the outlet ? 49. A gas stream containing acetone in air flows from a solvent recovery
please explain, why we said that is saturated in the outlet ? 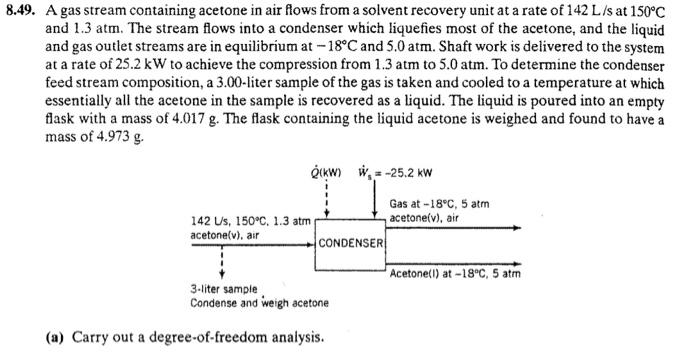
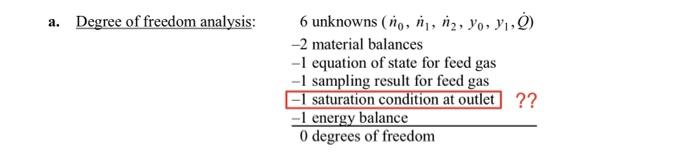
49. A gas stream containing acetone in air flows from a solvent recovery unit at a rate of 142L/s at 150C and 1.3atm. The stream flows into a condenser which liquefies most of the acetone, and the liquid and gas outlet streams are in equilibrium at 18C and 5.0atm. Shaft work is delivered to the system at a rate of 25.2kW to achieve the compression from 1.3atm to 5.0atm. To determine the condenser feed stream composition, a 3.00-liter sample of the gas is taken and cooled to a temperature at which essentially all the acetone in the sample is recovered as a liquid. The liquid is poured into an empty flask with a mass of 4.017g. The flask containing the liquid acetone is weighed and found to have a mass of 4.973g. (a) Carry out a degree-of-freedom analysis. a. Degree of freedom analysis: 6unknowns(n0,n1,n2,y0,y1,Q)2materialbalances1equationofstateforfeedgas1samplingresultforfeedgas1saturationconditionatoutlet0degreesoffreedom1energybalance 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


