Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please find kfinalfor both parts for part b what is the equillibrium constant for PbCl2 +2Ag^+ =2AgCl+Pb^2+ Review Constants Periodic Table 1. HS(aq) +HS (aq)
please find kfinalfor both parts 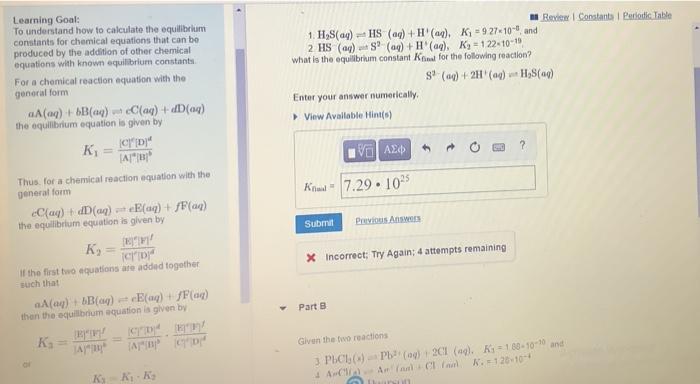

for part b what is the equillibrium constant for PbCl2 +2Ag^+ =2AgCl+Pb^2+
Review Constants Periodic Table 1. HS(aq) +HS (aq) + H(aq)K = 9 27-10-and 2 HS () SP (aq) + (aq). Ky - 122-10-15 what is the equilibrium constant Kit for the following reaction? S (0) + 2H (4) Hy8(a) Enter your answer numerically Viw Available Hint(s) ? V AED Learning Goal: To understand how to calculate the equilibrium constants for chemical equations that can be produced by the addition of other chemical equations with known equilibrium constant For a chemical reaction equation with the general form A(aq) + 6B() Caq) + (aq) the equilibrium equation is given by K CD" A1" Thus for a chemical reaction equation with the general form Cay) + dD(aq) + (aq) + f(a) the equilibrium equation is given by EW K If the first two equations are added together such that A(0) + B(aq) = (aq) + F(44) than the equilibrium equation is given by Knal = 7.29 105 Submit Previous Answers X Incorrect; Try Again: 4 attempts remaining Part B . Apm Given the two reactions 3 PCI) Pb(20) 201 (9). Ky = 189-10-10 and Ar land allan K. = 120-10-4 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


