Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please find the answer to the questions and fill in the tables (please indicate how you solved it) please find the answer to the questions
please find the answer to the questions and fill in the tables (please indicate how you solved it) 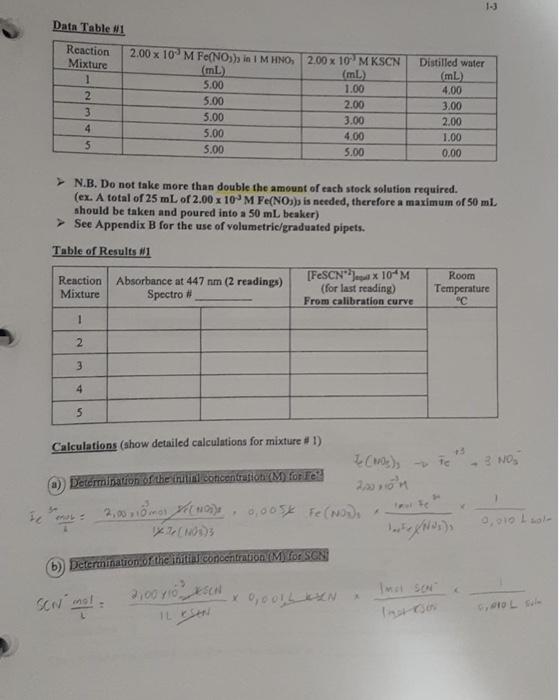



please find the answer to the questions and fill in the tables(and provide the solution to it) 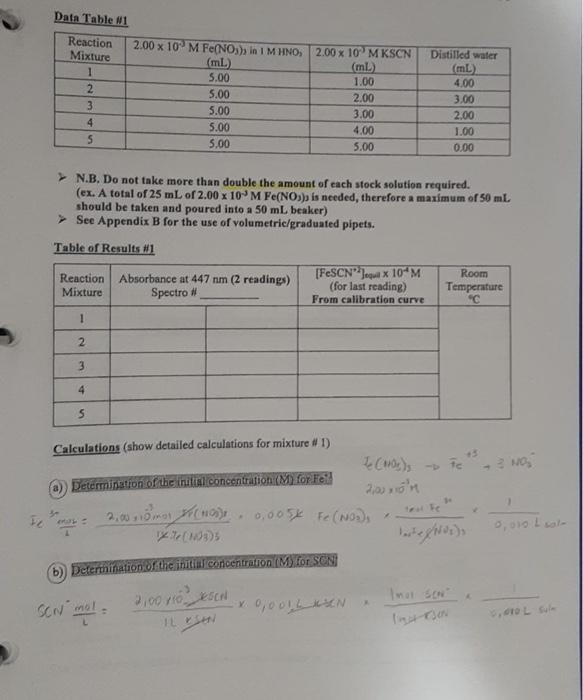
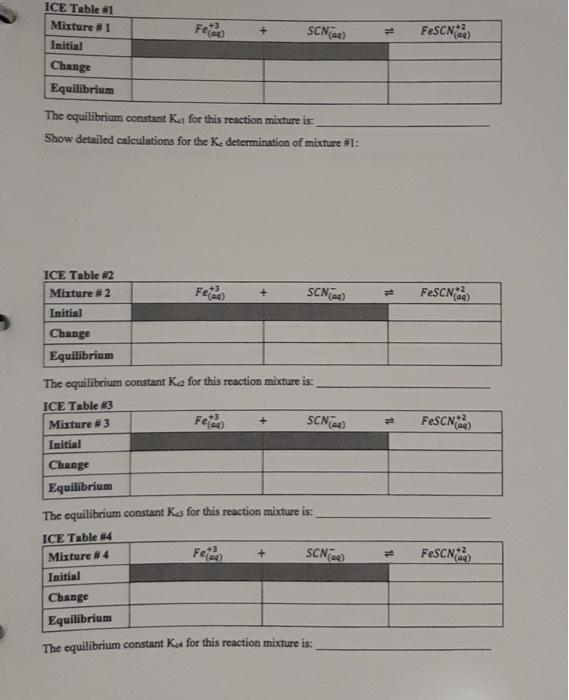
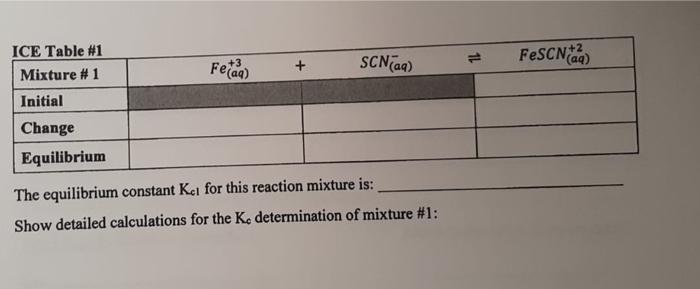
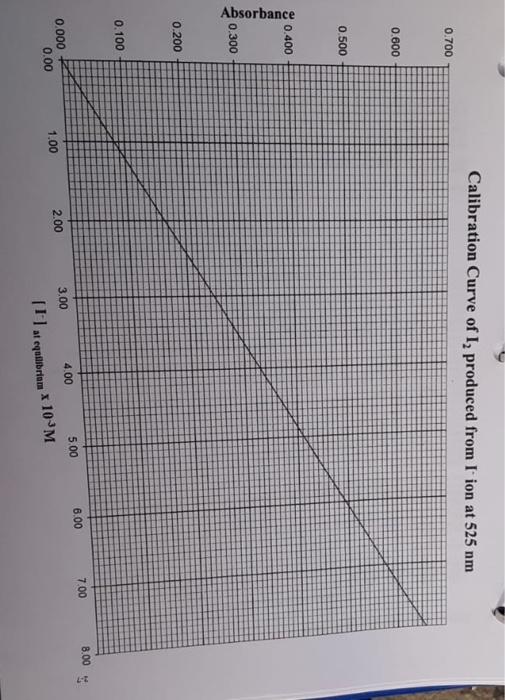
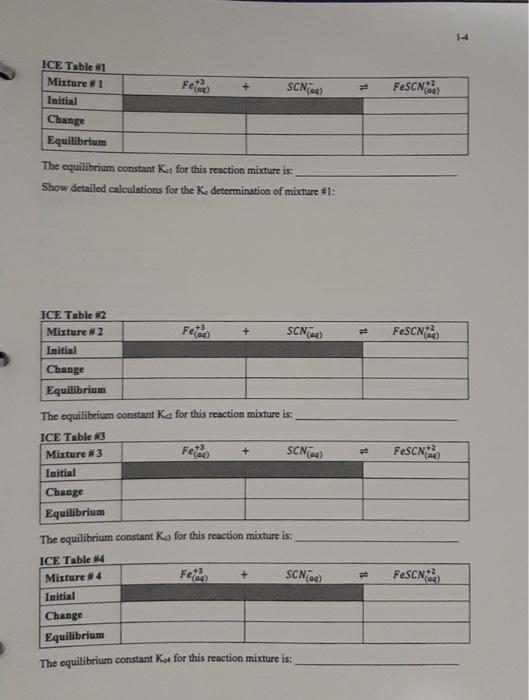
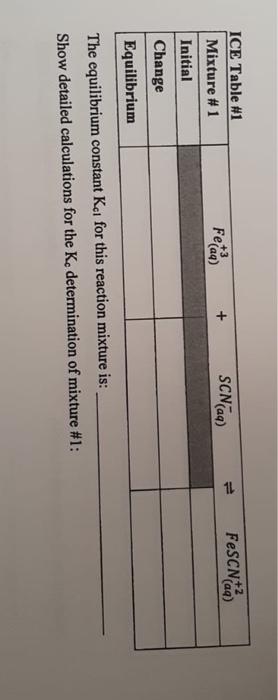
1-3 Data Table 1 Reaction Mixture 1 2 3 4 5 2.00 x 10 M Fe(NO) in 1 M HNO, 2.00 x 10 MKSCN (mL) (m 5.00 1.00 5.00 2.00 5.00 3.00 5.00 4.00 5.00 5.00 Distilled water (mL 4,00 3.00 2.00 1.00 0.00 N.B. Do not take more than double the amount of each stock solution required. (ex. A total of 25 mL of 2.00 x 10 M Fe(NO3)3 is needed, therefore a maximum of 50 ml. should be taken and poured into a 50 mL beaker) See Appendix B for the use of volumetric/graduated pipets. Table of Results 1 Room [FeSCNJ X 10 M Reaction Absorbance at 447 nm (2 readings) (for last reading) Temperature Mixture Spectro # From calibration curve 1 2 3 4 5 Calculations (show detailed calculations for mixture # 1) Cor) 3. NO (1) Determination of the initial concentration (M) for Te 2,021 v terte 6,0054 Fe(NODI 0.010 Lol- NOD3 b) Determination of the initial concentration (My for SON 2,000 KSON 12 ron Imo SEN son mot Intro COOL solo 14 Feide SCN) FeSCN2 ICE Table 1 Mixture1 Initial Change Equilibrium The equilibrium constant Kat for this reaction mixture is: Show detailed calculations for the Ke determination of mixture #1: ICE Table #2 Mixture #2 Initial Feed SCN) FeSCN) Change Equilibrium Feide FeSCN The equilibrium constant ka for this reaction mixture is: ICE Table #3 Mixture #3 SCNG) Initial Change Equilibrium The equilibrium constant Ka for this reaction mixture is: ICE Table 14 Misture #4 Initial Change Equilibrium The equilibrium constant Kot for this reaction mixture is: Feide SCN) FeSCN2 FeSCN +3 SCN) 1L + Fetaa) ICE Table #1 Mixture #1 Initial Change Equilibrium The equilibrium constant Kct for this reaction mixture is: Show detailed calculations for the Ke determination of mixture #1: Data Table #1 Reaction Mixture 1 2 3 4 S 2.00 x 10 M Fe(NO3), in 1 M HNO, 2.00 x 10 MKSCN (mL) (ml) 5.00 1.00 5.00 2.00 5.00 3.00 5.00 4.00 5.00 5.00 Distilled water (mL) 4,00 3.00 2.00 1.00 0.00 N.B. Do not take more than double the amount of each stock solution required. (ex. A total of 25 mL of 2.00 x 10 M Fe(NO))) is needed, therefore a maximum of 50 ml should be taken and poured into a 50 mL beaker) See Appendix B for the use of volumetric/graduated pipets. Table of Results #1 Reaction Mixture Absorbance at 447 nm (2 readings) Spectro # [FeSCNJeux 10-M (for last reading) From calibration curve Room Temperature "C 1 2 3 4 5 Calculations (show detailed calculations for mixture # 1) (o)s To NO Determination of the initial concentration (M) for Fel! 2,00 31 mayo 0,0054 Fo (NO) 1 Too Fe To 0,010 Isola 4 b) Determination of the initial concentration (M) for SONI SCN mola 2,001 est x 0,001LDEN ILUSH Inot son Intrs Feier SCN) FeSCN +2 (a) ICE Table 1 Mixture #1 Initial Change Equilibrium The equilibrium constant ket for this reaction mixture is Show detailed calculations for the determination of mixture #1: ICE Table #2 Mixture #2 Feed + SCND FeSCN) (4 Initial Change Equilibrium The equilibriurn constant Ke for this reaction mixture is: Feed + SCN) FeSCNO ICE Table #3 Mixture #3 Initial Change Equilibrium The equilibrium constant Ks for this reaction mixture is: ICE Table 4 Misture #4 Feme + Initial Change Equilibrium SCN) FeSON The equilibrium constant Kot for this reaction mixture is: +3 FeSCN) Fetaa) SCN) + ICE Table #1 Mixture # 1 Initial Change Equilibrium The equilibrium constant Kct for this reaction mixture is: Show detailed calculations for the Ke determination of mixture #1: Calibration Curve of I, produced from I ion at 525 nm 0.700 0.600 0.500 0.400 Absorbance 0.300 0.200 0.100 7.00 8.00 6.00 5.00 4.00 3.00 0.000 0.00 2.00 1.00 af equilibrium * 10 M 1-3 Data Table 1 Reaction Mixture 1 2 3 4 5 2.00 x 10 M Fe(NO) in 1 M HNO, 2.00 x 10 MKSCN (mL) (m 5.00 1.00 5.00 2.00 5.00 3.00 5.00 4.00 5.00 5.00 Distilled water (mL 4,00 3.00 2.00 1.00 0.00 N.B. Do not take more than double the amount of each stock solution required. (ex. A total of 25 mL of 2.00 x 10 M Fe(NO3)3 is needed, therefore a maximum of 50 ml. should be taken and poured into a 50 mL beaker) See Appendix B for the use of volumetric/graduated pipets. Table of Results 1 Room [FeSCNJ X 10 M Reaction Absorbance at 447 nm (2 readings) (for last reading) Temperature Mixture Spectro # From calibration curve 1 2 3 4 5 Calculations (show detailed calculations for mixture # 1) Cor) 3. NO (1) Determination of the initial concentration (M) for Te 2,021 v terte 6,0054 Fe(NODI 0.010 Lol- NOD3 b) Determination of the initial concentration (My for SON 2,000 KSON 12 ron Imo SEN son mot Intro COOL solo 14 Feide SCN) FeSCN2 ICE Table 1 Mixture1 Initial Change Equilibrium The equilibrium constant Kat for this reaction mixture is: Show detailed calculations for the Ke determination of mixture #1: ICE Table #2 Mixture #2 Initial Feed SCN) FeSCN) Change Equilibrium Feide FeSCN The equilibrium constant ka for this reaction mixture is: ICE Table #3 Mixture #3 SCNG) Initial Change Equilibrium The equilibrium constant Ka for this reaction mixture is: ICE Table 14 Misture #4 Initial Change Equilibrium The equilibrium constant Kot for this reaction mixture is: Feide SCN) FeSCN2 FeSCN +3 SCN) 1L + Fetaa) ICE Table #1 Mixture #1 Initial Change Equilibrium The equilibrium constant Kct for this reaction mixture is: Show detailed calculations for the Ke determination of mixture #1: Data Table #1 Reaction Mixture 1 2 3 4 S 2.00 x 10 M Fe(NO3), in 1 M HNO, 2.00 x 10 MKSCN (mL) (ml) 5.00 1.00 5.00 2.00 5.00 3.00 5.00 4.00 5.00 5.00 Distilled water (mL) 4,00 3.00 2.00 1.00 0.00 N.B. Do not take more than double the amount of each stock solution required. (ex. A total of 25 mL of 2.00 x 10 M Fe(NO))) is needed, therefore a maximum of 50 ml should be taken and poured into a 50 mL beaker) See Appendix B for the use of volumetric/graduated pipets. Table of Results #1 Reaction Mixture Absorbance at 447 nm (2 readings) Spectro # [FeSCNJeux 10-M (for last reading) From calibration curve Room Temperature "C 1 2 3 4 5 Calculations (show detailed calculations for mixture # 1) (o)s To NO Determination of the initial concentration (M) for Fel! 2,00 31 mayo 0,0054 Fo (NO) 1 Too Fe To 0,010 Isola 4 b) Determination of the initial concentration (M) for SONI SCN mola 2,001 est x 0,001LDEN ILUSH Inot son Intrs Feier SCN) FeSCN +2 (a) ICE Table 1 Mixture #1 Initial Change Equilibrium The equilibrium constant ket for this reaction mixture is Show detailed calculations for the determination of mixture #1: ICE Table #2 Mixture #2 Feed + SCND FeSCN) (4 Initial Change Equilibrium The equilibriurn constant Ke for this reaction mixture is: Feed + SCN) FeSCNO ICE Table #3 Mixture #3 Initial Change Equilibrium The equilibrium constant Ks for this reaction mixture is: ICE Table 4 Misture #4 Feme + Initial Change Equilibrium SCN) FeSON The equilibrium constant Kot for this reaction mixture is: +3 FeSCN) Fetaa) SCN) + ICE Table #1 Mixture # 1 Initial Change Equilibrium The equilibrium constant Kct for this reaction mixture is: Show detailed calculations for the Ke determination of mixture #1: Calibration Curve of I, produced from I ion at 525 nm 0.700 0.600 0.500 0.400 Absorbance 0.300 0.200 0.100 7.00 8.00 6.00 5.00 4.00 3.00 0.000 0.00 2.00 1.00 af equilibrium * 10 M 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


