Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please find the correct answers for the wrong ones! for the mass its 27.25 part C REPORT SUMMARY Table 5: Mass of liquid delivered by
please find the correct answers for the wrong ones!
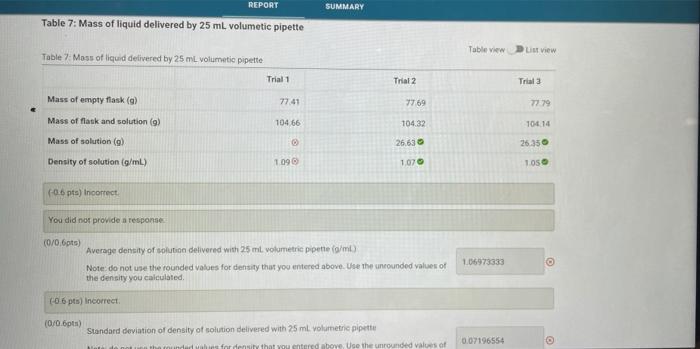
REPORT SUMMARY Table 5: Mass of liquid delivered by buret Table view LV Tables Mass at liquid delivered by bet Trial 1 Trial 2 Trial Mass of clean, dry Erlenmeyer flask (9) 78.06 7823 7021 Initial Buret Volume Reading (mi) 0.10 010 3.00 Buret Volume Reading after delivering -24 ml (ml) 2423 24.10 24.10 10416 104.92 101 Mass of flask and solution (9) Mass of solution delivered (9) 26.1080 26631 235540 1082 1.1090 11180 Density of solution (g/mL) 110320401 (06/06pts) Average density of solition delivered with bulut (g/ml) Note: do not use the rounded values for density that you entered above. Use the nounded values of the density you calculated (0/0 pts) Standard deviation of density of solution delivered with burit Note do not use the rounded values for density that you entered above. Use the unrounded values of 0.01534763 REPORT SUMMARY Table 7: Mass of liquid delivered by 25 ml. volumetic pipette Table view List View Table? Moss of liquid delivered by 25 mL volumetic pipette Trial 1 Trial 2 Trial Mass of empty flask (9) 77.41 7769 7779 Mass of flask and solution (9) 104.66 10432 10414 Mass of solution (9) 26.63 26.350 Density of solution (g/mL) 1.09 1070 TOS -06 pts) Incorrect You did not provide a response (0/06pts) Average density of solution delivered with 25 ml. volumetric pipette (g/ml) Note: do not use the rounded values for density that you entered above. Use the unrounded values of the density you calculated 1.06973333 06 pts) Incorrect (0/0.6pts) Standard deviation of density of solution delivered with 25 ml volumetric pipette for density that you entered above. Use the unrounded values of 0.07196554 for the mass its 27.25 part C 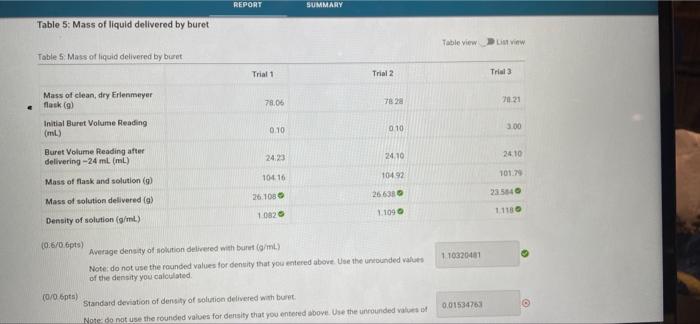


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


