Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help 100 90 80 NaNO3 70 60 CaCl2 KNO3 KCrO Solubility (g of salt in 100 g H,0) 50 Pb(NO3)2 KCI 40 NaCl 30
please help 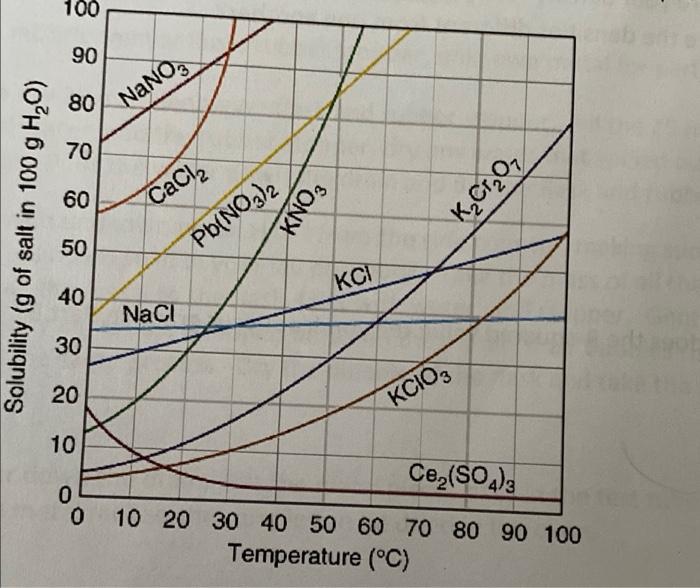


100 90 80 NaNO3 70 60 CaCl2 KNO3 KCrO Solubility (g of salt in 100 g H,0) 50 Pb(NO3)2 KCI 40 NaCl 30 20 KCIO, 10 Ce,(SO4)3 0 0 10 20 30 40 50 60 70 80 90 100 Temperature (C) 2. Using Figure 1, determine the solubility of KNO3 at 0C. 3. a. Using Figure 1, calculate the amount of water required to completely dissolve 25 g KCIO3 at 70C. b. Using Figure 1, what is the solubility of KClO3 at 0C? C. Using your answers from parts 3a and 3b, what amount of KCIO, originally added would remain in solution once cooled to 0C? d. What amount of KClo, originally added in question 3a should precipitate out of the solution once cooled to 0C. e. If the student recovered 15 grams of KClo, at the end of the experiment, what percentage of KCIO; was recovered? 4. Does increasing the temperature of all solids increase the solubility? 5. Referring to Figure 1, determine two solids that you could effectively separate (one would almost completely precipitate and one would stay in solution) at 40C if you added 40 g of both solids into 100 grams of water. 6. Referring to figure 1, if you added 50 g of each of the solids represented in the graph to a beaker and tried to dissolve them in 100 g of water at 90C, what solids would precipitate? (just name the solids, you do not need to calculate the quantity of solid that would precipitate) 


Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


