please-please help ASAP
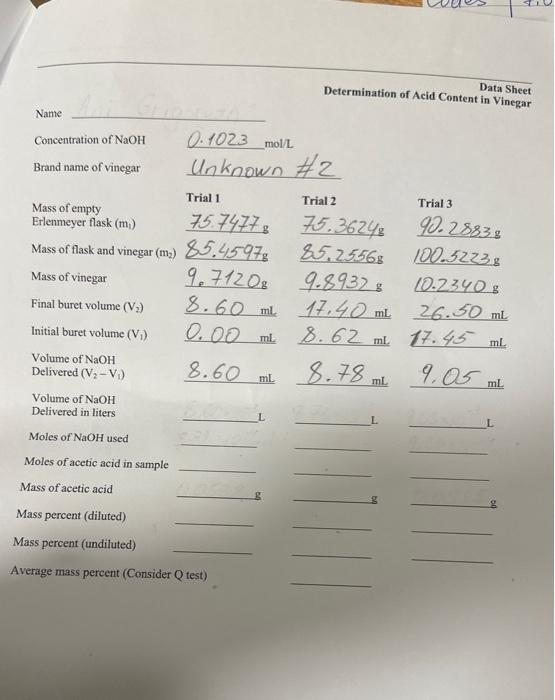
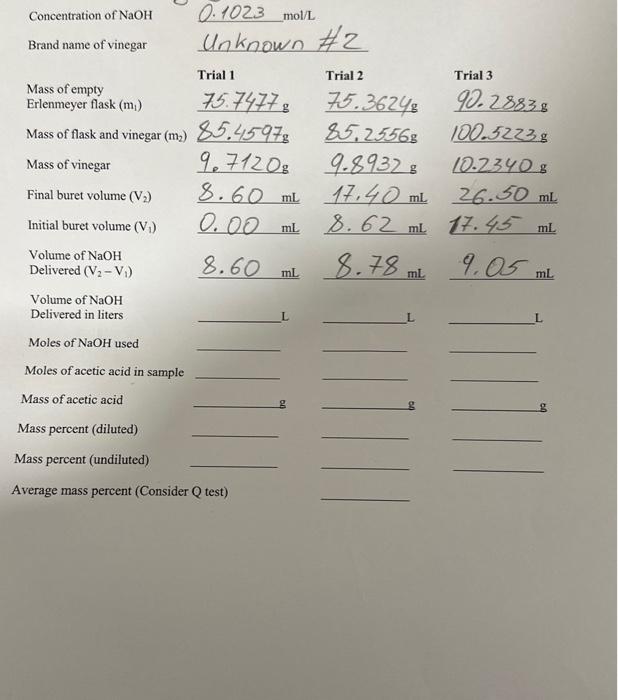
Concentration of NaOH0.1023mol/L Brand name of vinegar Unknown \#2 Initial buret volume (V1)0.00mL8.62mL27.43mL Volume of NaOH Delivered (V2V1) 8.60mL.78mL.05mL Volume of NaOH Delivered in liters Moles of NaOH used Moles of acetic acid in sample Mass of acetic acid Mass percent (diluted) Mass percent (undiluted) Average mass percent (Consider Q test) Name Determination of Acid Content in Sheet Concentration of NaOH0.102.3mol/L Brand name of vinegar lnknown 2 Initial buret volume (V1)0.00mL8.62mL.7.45mL Volume of NaOH Delivered (V2V1) 8.60mL.7.9mL.9mL Volume of NaOH Delivered in liters Moles of NaOH used Moles of acetic acid in sample Mass of acetic acid Mass percent (diluted) Mass percent (undiluted) Average mass percent (Consider Q test) The Determination of Acid Content in Vinegar Reading assignment: Chang, Chemistry 10th edition, pages 153-156. We will use a titration to determine the concentration of acctic acid in a sample of vinegar in order to become familiar with acid-base reactions. Digital analytical balance, 100-mL, beakers (2), 10-mL graduated cylinder, 100-mL, volumetric flask. 10-mL graduated piperte, pipette pump, 125-mL. Ericnmeyer flasks (3), 50 -mL, buret, phenolphthalein indicator, -0.1M sodrum hydroxide, vinegar. Safety Note: Safety glasses are required when performing this experiment. Acctic acid ( HC2H3O2) is the active ingredient in vincgar and is responsible for its sour taste. Acetic acid is an example of a wcak acid. For a 0.1molL solution of acetic acid only about 1% of the acid ionizes. Compare this to a strong acid like hydrochloric acid. Very close to 100% of hydrochloric acid ionizes. A few examples of strong and weak acids are shown below: Only one of the hydrogen atoms of the acetic acid molecule is acidic: Equation 1. HC2H1O2(aq)H(aq) acctic acid bydrogen ion acetais ion The structural formula for acctic acid is shown to the right. The bydrogen attached to the oxygen atom is acidie while the other hydrogen atoms are not. Another way of representing the acidity of acetic acid is to show its reaction with water: is very reactive and doesn't exist in water. However, there is evidence that the hydronium ion does exist. Sometimes cquation 1 is used because of its simplicity. To determine the amount of acetic acid in vinegar (typically 45% by mass) we will use an seid-base titration (neutralization reaction). In this experiment we titnate scetic acid with sodium hydroxide (a strong bafe). "The reaction of acctic acid with sodium hydroxide is shown bolow: Bquation 3. HC2H7O2(aq)+NaOH(aq)NaC2H3O2(aq)+ acelic acad sodium bydroxude sodium acctate In the reaction between acetic acid and sodium hydroxide, the acctic acid donates a proton to the hydroxide ion and acts as an acid. The hydroxide ion accepts a proton and acts as a base. The stoichiometric relationship between acetic acid and sodium hydroside is I:I (from Equation 2). If the number of moles of NaOH used to titrate a sample of acetic acid are known, then the moles of acetic acid in a sample can easily be found. Evidence of the reaction between acetic acid sodium hydroxide isn't visible, so we will add an indicator to determine the equivalence point for the titration. A common acid-base indicator is phenolphthalcin. Phenolphthalein is colorless in acidic so changes to pink when the solution beconses basic. At thei point, the moles of acetic acid are equal to the moles of s0 and the solution will tum pink as more sodium hydroxide Phenolphthalein is itself a weak acid. So when cnough so has been added to the acetic acid, such that there is no longer any unreacted acetic acid, the soditm bydroxide will then begin reacting with phenolphthalein. The reaction of plienolphthalein with sodium bydroxide results in a pink solution. The pictare abowe shows a titration apparatus. The experiment makes use of a buret that contains the sodium hydroxide solution. An Erleemeyer flask contains the vincgar solution and a couple of drops of the indicator (phenolphthalein). The sodium hydroxide solution is poured into the vinegar solution one drop at a time. The volume of rodium hydroxide required to react with all of the acetic acid in the vinegar is measured from the burct. Sample Calculation A 25.00ml, sample of a hydrofluoric acid, a monoprotic acid, is titrated with a 0.155M solution of sodium hydroxide. It is found that the indicator changes color once 31.25ml. of the sodium hydroxide solueion has becn added to the acid. What is the molarity of the acid? The equation for the reaction is HF(aq +NaOH(aq)NaF(aq)+H2OCi) At the equivalenee point the moles of the acid (HF) are equal to the moles of the base (NaOH).* We cain use the known concentration and measured volume of the sodiam hydroxide to find the number of moles of bydroxide used in the titration: moles NaOH =(1L0.155molNaOH)(0.03125LNaOH)=0.00484molNaOH where the volume has been converted to liters. At the equivalence point: mol HE molNzOH so moles of HF=0.00484mol The eonceatration of the acid is equal to the number of moles divided by its volume: molarify of acid =0.02500L0.00484molHF=0.194molL "The solution chianger color once all of the acid has reacted with the base and souse of the indicator reacts with the base. So the change in color otten occurs at w bat is called the erdpeint, a little beyond the equivalence point. 2 SAFETY PRECAUTIONS Acetic acid and sodium hydroxide are both irritants. Safety glasses must be wom at all times during this experiment. Students perform titrations individually. 1. Obtain about 60mL of sodium hydroxide (NaOH) in a clean dry beaker. Be sure to write down the concentration of the sodium hydroxide from the bottle (or from the board at the front of the room). 2. Prepare the buret by rinsing the buret with tap water, then with distilled or deionized water, and finally with about 5mL of sodium hydroxide solution. 3. Fill the buret with sodium hydroxide solution to a level slightly above the 0mL mark. Open the valve on the buret and allow a couple milliliters to flow into a waste beaker so that any air bubbles can be forced through. Record the volume of the sodium hydroxide in the buret on the data shest (initial volume). 4. The vinegar we will be using has an acetic acid concentration that would require a large volume of sodium hydroxide for this titration. So we will dilute the vinegar with water before performing the titration. Obtain 15-20 mL of vinegar in a clean and dry 100mL beaker. Record the brand name of the vinegar. 5. Using a 10 -mL volumetric pipette carefully pipet 10mL of vinegar into a 100 -mL volumetric flask. 6. Fill the flask to the 100mL mark using distilled or deionized water. Use this diluted vinegar to perform the titrations. 7. Measure the mass of 3 clean 125 -mL. Erlenmeyer flasks (individually) on the digital analytical balance to the nearest 0.1mg(0.0001g). Record each mass on the data sheet. Erlenmeyer flasks. 9. Measure the mass of each of the Erlenmeyer flasks containing the diluted vinegar. Record these masses on the data sheet. 10. Add two drops of phenolphthalein indicator to each Erlenmeyer flask containing dilute vinegar and begin the titration by silowly adding the sodium hydroxide solution drop by drop from the buret to the flask. The solution should initially be clear, with no pink tint. Be sure to swirl the solution in the flask while adding the sodium hydroxide solution. The endpoint for this titration is a very faint pink color that penists for more than 15 seconds. Darker pink colors indicate that you have added too much sodium hydroxide. Read the volume to 0.02mL. 11. Perform the titration a minimum of three times until you have three sodium hydroxide volumes that are within 0.20mL of each other. You can perform additional titrations if your delivered volumes are not close to one another. 12. Waste can be disposed of in the sink. Clean your work area before beginning calculations