Answered step by step
Verified Expert Solution
Question
1 Approved Answer
At 30.0C, the molar solubility of barium sulfate in water is 1.20x10-5 M. Calculate the solubility in grams per liter. Express your answer in
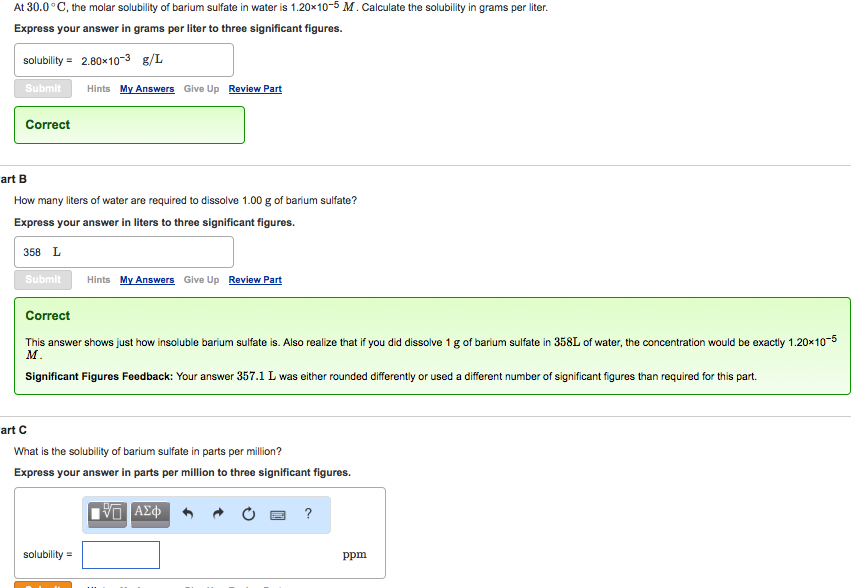
At 30.0C, the molar solubility of barium sulfate in water is 1.20x10-5 M. Calculate the solubility in grams per liter. Express your answer in grams per liter to three significant figures. solubility = 2.8010-3 g/L Submit Correct Hints My Answers Give Up Review Part art B How many liters of water are required to dissolve 1.00 g of barium sulfate? Express your answer in liters to three significant figures. 358 L Submit Hints My Answers Give Up Review Part Correct This answer shows just how insoluble barium sulfate is. Also realize that if you did dissolve 1 g of barium sulfate in 358L of water, the concentration would be exactly 1.20x10-5 M. Significant Figures Feedback: Your answer 357.1 L was either rounded differently or used a different number of significant figures than required for this part. solubility= art C What is the solubility of barium sulfate in parts per million? Express your answer in parts per million to three significant figures. ? ppm
Step by Step Solution
★★★★★
3.39 Rating (152 Votes )
There are 3 Steps involved in it
Step: 1
PART C relation between solubilit...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


