Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help from 1 to 4 1. What is the concentration of ClO41 ion in a 0.200mol/L solution of Ti(ClO4)4 ? (A) 0.600mol/L (B) 0.800mol/L
please help from 1 to 4 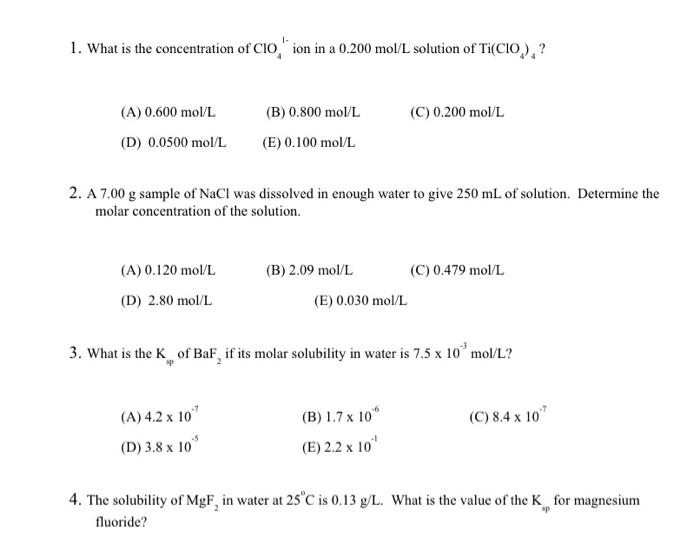
1. What is the concentration of ClO41 ion in a 0.200mol/L solution of Ti(ClO4)4 ? (A) 0.600mol/L (B) 0.800mol/L (C) 0.200mol/L (D) 0.0500mol/L (E) 0.100mol/L 2. A 7.00g sample of NaCl was dissolved in enough water to give 250mL of solution. Determine the molar concentration of the solution. (A) 0.120mol/L (B) 2.09mol/L (C) 0.479mol/L (D) 2.80mol/L (E) 0.030mol/L 3. What is the Ksp of BaF2 if its molar solubility in water is 7.5103mol/L ? (A) 4.2107 (B) 1.7106 (C) 8.4107 (D) 3.8105 (E) 2.2101 4. The solubility of MgF2 in water at 25C is 0.13g/L. What is the value of the Ksp for magnesium fluoride 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


