Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help I have limited time !! A 37.5 g sample of copper at 99.8 C is carefully placed into an insulated container containing 1919
please help I have limited time !! 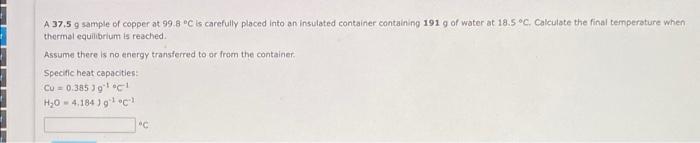

A 37.5 g sample of copper at 99.8 C is carefully placed into an insulated container containing 1919 of water at 18.5 C. Calculate the final temperature when thermal equilibrium is reached Assume there is no energy transferred to or from the container, Specific heat capacities CU = 0.385 Joc H20 - 4,184 ) How much energy must be transferred to raise the temperature of a cup of coffee (250 ml) from 20.6 C (293.75 K) to 95.0 C (368.15 K)? Assume that water and coffee have the same density (1,00 g/mL) and specific heat capacity (4.184 J/gK). Submit Show Approach Show Tutor Stens 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


