Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help im really stuck When species combine to produce a coordination complex, the equilibrium constant for the reaction is called is the formation constant,
please help im really stuck 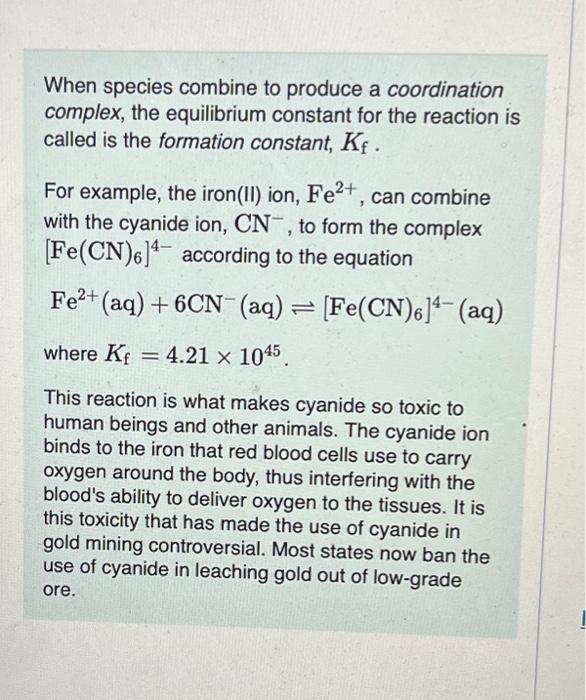
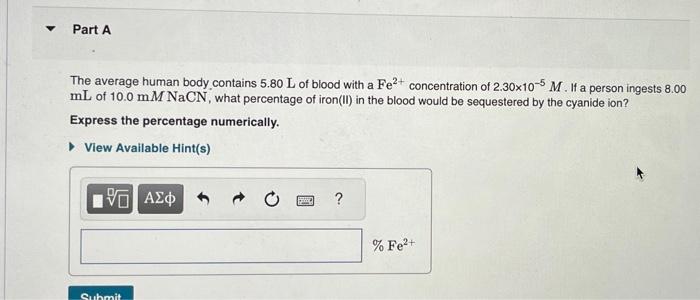

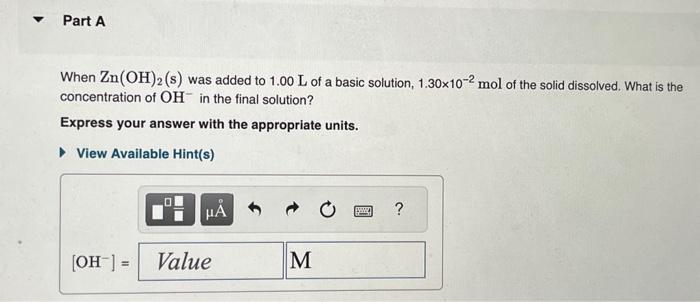
When species combine to produce a coordination complex, the equilibrium constant for the reaction is called is the formation constant, Kf. For example, the iron(II) ion, Fe2+, can combine with the cyanide ion, CN, to form the complex [Fe(CN)6]4 according to the equation Fe2+(aq)+6CN(aq)[Fe(CN)6]4(aq) where Kf=4.211045. This reaction is what makes cyanide so toxic to human beings and other animals. The cyanide ion binds to the iron that red blood cells use to carry oxygen around the body, thus interfering with the blood's ability to deliver oxygen to the tissues. It is this toxicity that has made the use of cyanide in gold mining controversial. Most states now ban the use of cyanide in leaching gold out of low-grade ore. The average human body contains 5.80L of blood with a Fe2+ concentration of 2.30105M. If a person ingests 8.00 mL of 10.0mMNaCN, what percentage of iron(II) in the blood would be sequestered by the cyanide ion? Express the percentage numerically. The solubility-product constant for Zn(OH)2 is Ksp=3.001016. The formation constant for the hydroxo complex, Zn(OH)42, is Kf=4.601017. When Zn(OH)2(s) was added to 1.00L of a basic solution, 1.30102mol of the solid dissolved. What is the concentration of OHin the final solution? Express your answer with the appropriate units 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


