Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help me quickly Question 3 1 Point Ferrocene, [Fe(C10H10)(s) molar mass 186.03 g/mol]can be prepared by reacting 6.0 g of FeCl2(S) (molar mass =126.75
please help me quickly 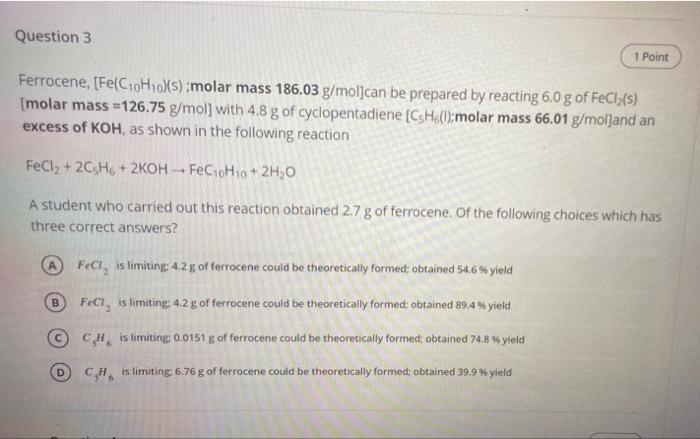
Question 3 1 Point Ferrocene, [Fe(C10H10)(s) molar mass 186.03 g/mol]can be prepared by reacting 6.0 g of FeCl2(S) (molar mass =126.75 g/mol] with 4.8 g of cyclopentadiene (CsHoU;molar mass 66.01 g/molland an excess of KOH, as shown in the following reaction FeCl2 + 2C5H + 2KOH -- FeC10H1o + 2H,0 A student who carried out this reaction obtained 2.7 g of ferrocene of the following choices which has three correct answers? FeCl, is limiting 4.2 g of ferrocene could be theoretically formed obtained 54.6% yield B FeCl, is limiting: 4.2 g of ferrocene could be theoretically formed; obtained 89.4 yield C# is limiting: 0.0151 g of ferrocene could be theoretically formed, obtained 74.8 % yield C.H. is limiting 6.76 g of ferrocene could be theoretically formed obtained 39.9 yield D 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


