Answered step by step
Verified Expert Solution
Question
1 Approved Answer
please help to answer all the questions: 1) a) i,ii,iii,iv,v,vi,vii, and b) i,ii. give me the details, answer, and hints, please as soon as possible!
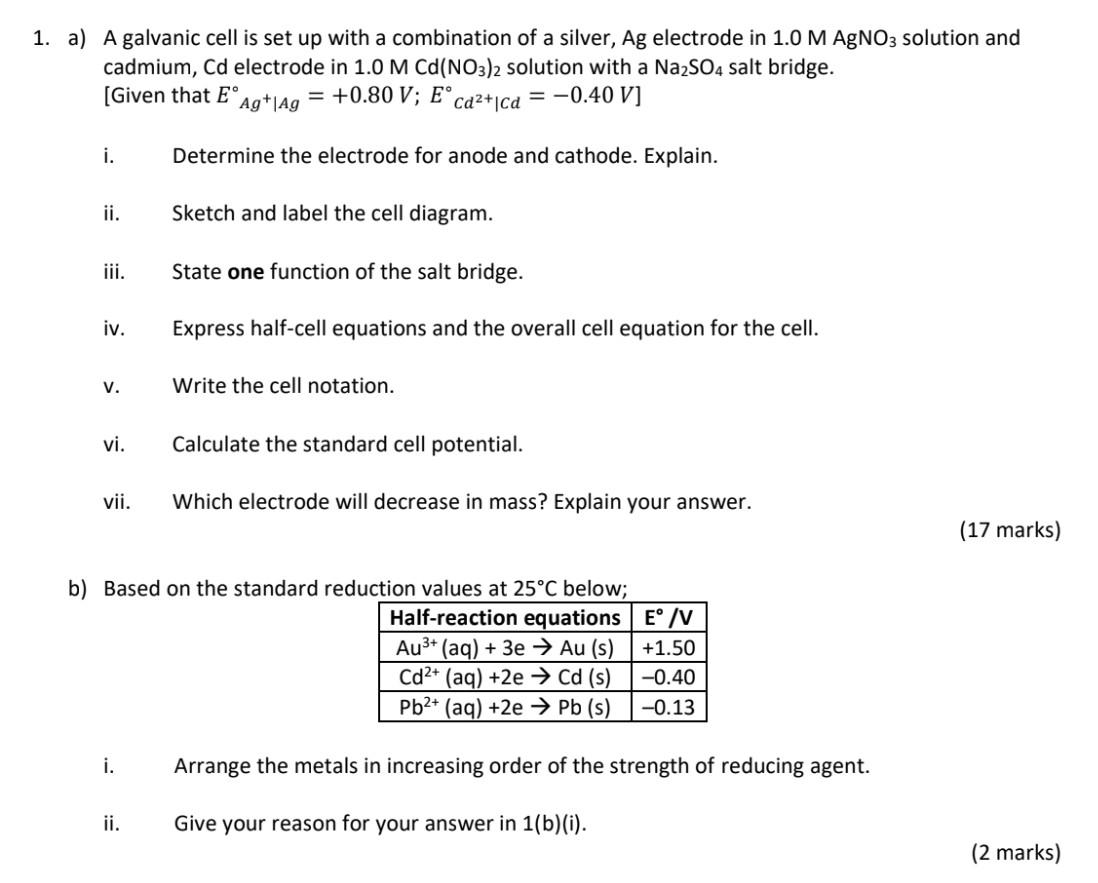
please help to answer all the questions: 1) a) i,ii,iii,iv,v,vi,vii, and b) i,ii. give me the details, answer, and hints, please as soon as possible!
1. a) A galvanic cell is set up with a combination of a silver, Ag electrode in 1.0MAgNO3 solution and cadmium, Cd electrode in 1.0MCd(NO3)2 solution with a Na2SO4 salt bridge. [Given that EAgAg=+0.80V;ECd2+Cd=0.40V ] i. Determine the electrode for anode and cathode. Explain. ii. Sketch and label the cell diagram. iii. State one function of the salt bridge. iv. Express half-cell equations and the overall cell equation for the cell. v. Write the cell notation. vi. Calculate the standard cell potential. vii. Which electrode will decrease in mass? Explain your answer. (17 marks) b) Based on the standard reduction values at 25C below; i. Arrange the metals in increasing order of the strength of reducing agent. ii. Give your reason for your answer in 1 (b)(i). (2 marks)Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


